Sildenafil Citrate
These highlights do not include all the information needed to use SILDENAFIL TABLETS safely and effectively. See full prescribing information for SILDENAFIL TABLETS. SILDENAFIL tablets, for oral use Initial U.S. Approval: 1998
6d8dbaa6-d61a-46ae-a641-f664104f4714
HUMAN PRESCRIPTION DRUG LABEL
Sep 5, 2023
ANI Pharmaceuticals, Inc.
DUNS: 145588013
Products 3
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Sildenafil Citrate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Sildenafil Citrate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Sildenafil Citrate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Sildenafil Tablets, USP 25 mg - NDC-70954-407-10 - Bottles of 30

Sildenafil Tablets, USP 50 mg - NDC-70954-408-10 - Bottles of 30

Sildenafil Tablets, USP 100 mg - NDC-70954-409-10 - Bottles of 30

CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
4.1 Nitrates
Consistent with its known effects on the nitric oxide/cGMP pathway [see Clinical Pharmacology (12.1, 12.2)], sildenafil was shown to potentiate the hypotensive effects of nitrates, and its administration to patients who are using nitric oxide donors such as organic nitrates or organic nitrites in any form either regularly and/or intermittently is therefore contraindicated.
After patients have taken sildenafil, it is unknown when nitrates, if necessary, can be safely administered. Although plasma levels of sildenafil at 24 hours post dose are much lower than at peak concentration, it is unknown whether nitrates can be safely co-administered at this time point [see Dosage and Administration (2.3), Drug Interactions (7.1), and Clinical Pharmacology (12.2)].
4.2 Hypersensitivity Reactions
Sildenafil is contraindicated in patients with a known hypersensitivity to sildenafil, as contained in sildenafil and REVATIO, or any component of the tablet. Hypersensitivity reactions have been reported, including rash and urticaria [see Adverse Reactions (6.1)].
4.3 Concomitant Guanylate Cyclase (GC) Stimulators
Do not use sildenafil in patients who are using a GC stimulator, such as riociguat. PDE5 inhibitors, including sildenafil, may potentiate the hypotensive effects of GC stimulators.
- Administration of sildenafil to patients using nitric oxide donors, such as organic nitrates or organic nitrites in any form. Sildenafil was shown to potentiate the hypotensive effect of nitrates (4.1, 7.1, 12.2)
- Known hypersensitivity to sildenafil or any component of tablet (4.2)
- Administration with guanylate cyclase (GC) stimulators, such as riociguat (4.3)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following are discussed in more detail in other sections of the labeling:
- Cardiovascular [see Warnings and Precautions (5.1)]
- Prolonged Erection and Priapism [see Warnings and Precautions (5.2)]
- Effects on the Eye [see Warnings and Precautions (5.3)]
- Hearing Loss [see Warnings and Precautions (5.4)]
- Hypotension when Co-administered with Alpha-blockers or Anti-hypertensives [seeWarnings and Precautions (5.5)]
- Adverse Reactions with the Concomitant Use of Ritonavir [see Warnings andPrecautions (5.6)]
- Combination with other PDE5 Inhibitors or Other Erectile Dysfunction Therapies [seeWarnings and Precautions (5.7)]
- Effects on Bleeding [see Warnings and Precautions (5.8)]
- Counseling Patients About Sexually Transmitted Diseases [see Warnings and Precautions (5.9)]
The most common adverse reactions reported in clinical trials (≥ 2%) are headache, flushing, dyspepsia, abnormal vision, nasal congestion, back pain, myalgia, nausea, dizziness, and rash.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Sildenafil was administered to over 3700 patients (aged 19–87 years) during premarketing clinical trials worldwide. Over 550 patients were treated for longer than one year.
In placebo-controlled clinical studies, the discontinuation rate due to adverse reactions for sildenafil (2.5%) was not significantly different from placebo (2.3%).
In fixed-dose studies, the incidence of some adverse reactions increased with dose. The type of adverse reactions in flexible-dose studies, which reflect the recommended dosage regimen, was similar to that for fixed-dose studies. At doses above the recommended dose range, adverse reactions were similar to those detailed in Table 1 below but generally were reported more frequently.
Table 1: Adverse Reactions Reported by ≥2% of Patients Treated with Sildenafil and More Frequent than Placebo in Fixed-Dose Phase II/III Studies
|
Adverse Reaction |
25 mg |
50 mg |
100 mg |
Placebo |
|
Headache |
16% |
21% |
28% |
7% |
|
Flushing |
10% |
19% |
18% |
2% |
|
Dyspepsia |
3% |
9% |
17% |
2% |
|
Abnormal vision* |
1% |
2% |
11% |
1% |
|
Nasal congestion |
4% |
4% |
9% |
2% |
|
Back pain |
3% |
4% |
4% |
2% |
|
Myalgia |
2% |
2% |
4% |
1% |
|
Nausea |
2% |
3% |
3% |
1% |
|
Dizziness |
3% |
4% |
3% |
2% |
|
Rash |
1% |
2% |
3% |
1% |
*Abnormal Vision: Mild to moderate in severity and transient, predominantly color tinge to vision, but also increased sensitivity to light, or blurred vision.
When sildenafil was taken as recommended (on an as-needed basis) in flexible- dose, placebo-controlled clinical trials of two to twenty-six weeks duration, patients took sildenafil at least once weekly, and the following adverse reactions were reported:
Table 2. Adverse Reactions Reported by ≥2% of Patients Treated with Sildenafil and More Frequent than Placebo in Flexible-Dose Phase II/III Studies
|
Adverse Reaction |
Sildenafil |
PLACEBO |
|
Headache |
16% |
4% |
|
Flushing |
10% |
1% |
|
Dyspepsia |
7% |
2% |
|
Nasal Congestion |
4% |
2% |
|
Abnormal Vision* |
3% |
0% |
|
Back pain |
2% |
2% |
|
Dizziness |
2% |
1% |
|
Rash |
2% |
1% |
*Abnormal Vision: Mild and transient, predominantly color tinge to vision, but also increased sensitivity to light or blurred vision. In these studies, only one patient discontinued due to abnormal vision.
The following events occurred in < 2% of patients in controlled clinical trials; a causal relationship to sildenafil is uncertain. Reported events include those with a plausible relation to drug use; omitted are minor events and reports too imprecise to be meaningful:
Body as a Whole: face edema, photosensitivity reaction, shock, asthenia, pain, chills, accidental fall, abdominal pain, allergic reaction, chest pain, accidental injury.
Cardiovascular: angina pectoris, AV block, migraine, syncope, tachycardia, palpitation, hypotension, postural hypotension, myocardial ischemia, cerebral thrombosis, cardiac arrest, heart failure, abnormal electrocardiogram, cardiomyopathy.
Digestive: vomiting, glossitis, colitis, dysphagia, gastritis, gastroenteritis, esophagitis, stomatitis, dry mouth, liver function tests abnormal, rectal hemorrhage, gingivitis.
Hemic and Lymphatic: anemia and leukopenia.
Metabolic and Nutritional: thirst, edema, gout, unstable diabetes, hyperglycemia, peripheral edema, hyperuricemia, hypoglycemic reaction, hypernatremia.
Musculoskeletal: arthritis, arthrosis, myalgia, tendon rupture, tenosynovitis, bone pain, myasthenia, synovitis.
Nervous: ataxia, hypertonia, neuralgia, neuropathy, paresthesia, tremor, vertigo, depression, insomnia, somnolence, abnormal dreams, reflexes decreased, hypesthesia.
Respiratory: asthma, dyspnea, laryngitis, pharyngitis, sinusitis, bronchitis, sputum increased, cough increased.
Skin and Appendages: urticaria, herpes simplex, pruritus, sweating, skin ulcer, contact dermatitis, exfoliative dermatitis.
Special Senses: sudden decrease or loss of hearing, mydriasis, conjunctivitis, photophobia, tinnitus, eye pain, ear pain, eye hemorrhage, cataract, dry eyes.
Urogenital: cystitis, nocturia, urinary frequency, breast enlargement, urinary incontinence, abnormal ejaculation, genital edema and anorgasmia.
Analysis of the safety database from controlled clinical trials showed no apparent difference in adverse reactions in patients taking sildenafil with and without antihypertensive medication. This analysis was performed retrospectively, and was not powered to detect any pre-specified difference in adverse reactions.
6.2 Post-marketing Experience
The following adverse reactions have been identified during post approval use of sildenafil. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These events have been chosen for inclusion either due to their seriousness, reporting frequency, lack of clear alternative causation, or a combination of these factors.
Cardiovascular and cerebrovascular
Serious cardiovascular, cerebrovascular, and vascular events, including myocardial infarction, sudden cardiac death, ventricular arrhythmia, cerebrovascular hemorrhage, transient ischemic attack, hypertension, subarachnoid and intracerebral hemorrhages, and pulmonary hemorrhage have been reported post-marketing in temporal association with the use of sildenafil. Most, but not all, of these patients had preexisting cardiovascular risk factors. Many of these events were reported to occur during or shortly after sexual activity, and a few were reported to occur shortly after the use of sildenafil without sexual activity. Others were reported to have occurred hours to days after the use of sildenafil and sexual activity. It is not possible to determine whether these events are related directly to sildenafil, to sexual activity, to the patient's underlying cardiovascular disease, to a combination of these factors, or to other factors [see Warnings and Precautions (5.1) and Patient Counseling Information (17)].
**Hemic and Lymphatic:**vaso-occlusive crisis: In a small, prematurely terminated study of REVATIO (Sildenafil) in patients with pulmonary arterial hypertension (PAH) secondary to sickle cell disease, vaso-occlusive crises requiring hospitalization were more commonly reported in patients who received sildenafil than in those randomized to placebo. The clinical relevance of this finding to men treated with sildenafil for ED is not known.
Nervous:seizure, seizure recurrence, anxiety, and transient global amnesia.
**Respiratory:**epistaxis
Special senses:
Hearing: Cases of sudden decrease or loss of hearing have been reported post-marketing in temporal association with the use of PDE5 inhibitors, including sildenafil. In some of the cases, medical conditions and other factors were reported that may have also played a role in the otologic adverse events. In many cases, medical follow-up information was limited. It is not possible to determine whether these reported events are related directly to the use of sildenafil, to the patient's underlying risk factors for hearing loss, a combination of these factors, or to other factors [see Warnings and Precautions (5.4) and Patient Counseling Information (17)].
Ocular**:** diplopia, temporary vision loss/decreased vision, ocular redness or bloodshot appearance, ocular burning, ocular swelling/pressure, increased intraocular pressure, retinal edema, retinal vascular disease or bleeding, and vitreous traction/detachment.
Non-arteritic anterior ischemic optic neuropathy (NAION), a cause of decreased vision including permanent loss of vision, has been reported rarely post- marketing in temporal association with the use of phosphodiesterase type 5 (PDE5) inhibitors, including sildenafil. Most, but not all, of these patients had underlying anatomic or vascular risk factors for developing NAION, including but not necessarily limited to: low cup to disc ratio ("crowded disc"), age over 50, diabetes, hypertension, coronary artery disease, hyperlipidemia and smoking [see Warnings and Precautions (5.3) and Patient Counseling Information (17)].
Urogenital: prolonged erection, priapism [see Warnings and Precautions (5.2) and Patient Counseling Information (17)], and hematuria.
Most common adverse reactions (≥ 2%) include headache, flushing, dyspepsia, abnormal vision, nasal congestion, back pain, myalgia, nausea, dizziness and rash (6.1) (6)
To report SUSPECTED ADVERSE REACTIONS, contact Novitium Pharma LLC at 1-855-204-1431 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. (6)
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE & ADMINISTRATION
2.1 Dosage Information
For most patients, the recommended dose is 50 mg taken, as needed, approximately 1 hour before sexual activity. However, Sildenafil may be taken anywhere from 30 minutes to 4 hours before sexual activity.
The maximum recommended dosing frequency is once per day.
Based on effectiveness and toleration, the dose may be increased to a maximum recommended dose of 100 mg or decreased to 25 mg.
2.2 Use with Food
Sildenafil may be taken with or without food.
2.3 Dosage Adjustments in Specific Situations
Sildenafil was shown to potentiate the hypotensive effects of nitrates and its administration in patients who use nitric oxide donors such as organic nitrates or organic nitrites in any form is therefore contraindicated [see Contraindications (4.1), Drug Interactions (7.1), and Clinical Pharmacology (12.2)].
When sildenafil is co-administered with an alpha-blocker, patients should be stable on alpha-blocker therapy prior to initiating sildenafil treatment and sildenafil should be initiated at 25 mg [see Warnings and Precautions (5.5), Drug Interactions (7.2), and Clinical Pharmacology (12.2)].
2.4 Dosage Adjustments Due to Drug Interactions
Ritonavir
The recommended dose for ritonavir-treated patients is 25 mg prior to sexual activity and the recommended maximum dose is 25 mg within a 48-hour period because concomitant administration increased the blood levels of sildenafil by 11-fold [see Warnings and Precautions (5.6), Drug Interactions (7.4), and Clinical Pharmacology (12.3)].
CYP3A4 Inhibitors
Consider a starting dose of 25 mg in patients treated with strong CYP3A4 inhibitors (e.g., ketoconazole, itraconazole, or saquinavir) or erythromycin. Clinical data have shown that co-administration with saquinavir or erythromycin increased plasma levels of sildenafil by about 3-fold [see Drug Interactions (7.4) and Clinical Pharmacology (12.3)].
2.5 Dosage Adjustments in Specfic Populations
Consider a starting dose of 25 mg in patients > 65 years, patients with hepatic impairment (e.g., cirrhosis), and patients with severe renal impairment (creatinine clearance < 30 mL/minute) because administration of sildenafil in these patients resulted in higher plasma levels of sildenafil [see Use in Specific Populations (8.5, 8.6, 8.7) and Clinical Pharmacology (12.3)].
DESCRIPTION SECTION
11 DESCRIPTION
Sildenafil citrate, an oral therapy for erectile dysfunction, is the citrate salt of sildenafil, a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 (PDE5).
Sildenafil citrate is designated chemically as 1-[[3-(6,7-dihydro-1-methyl-7-oxo-3-propyl-1H-pyrazolo[4,3-d] pyrimidin-5-yl)-4-ethoxyphenyl] sulfonyl]-4-methylpiperazine citrate and has the following structural formula:
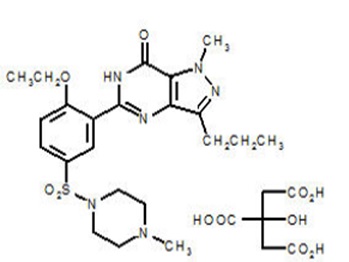
Sildenafil citrate is a white to off-white crystalline powder with a solubility of 3.5 mg/mL in water and a molecular weight of 666.7.
Sildenafil Tablets, USP are formulated as round shaped, blue colored (mottled), biconvex, uncoated, tablets equivalent to 25 mg, 50 mg and 100 mg of sildenafil for oral administration. In addition to the active ingredient, sildenafil citrate, each tablet contains the following inactive ingredients: lactose monohydrate, microcrystalline cellulose, anhydrous dibasic calcium phosphate, sucralose, peppermint flavor (arabic gum & natural flavors), bubblegum flavor (arabic gum, natural & artificial flavors), colloidal silicon dioxide, croscarmellose sodium, magnesium stearate, FD&C blue no. 1 aluminum lake, povidone.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Sildenafil was not carcinogenic when administered to rats for 24 months at a dose resulting in total systemic drug exposure (AUCs) for unbound sildenafil and its major metabolite of 20- and 38- times, for male and female rats, respectively, the exposures observed in human males given the Maximum Recommended Human Dose (MRHD) of 100 mg. Sildenafil was not carcinogenic when administered to mice for 18–21 months at dosages up to the Maximum Tolerated Dose (MTD) of 10 mg/kg/day, approximately 0.4 times the MRHD on a mg/m2 basis in a 50 kg subject.
Mutagenesis
Sildenafil was negative in in vitro bacterial and Chinese hamster ovary cell assays to detect mutagenicity, and in vitro human lymphocytes and in vivo mouse micronucleus assays to detect clastogenicity.
Impairment of Fertility
There was no impairment of fertility in rats given sildenafil up to 60 mg/kg/day for 36 days to females and 102 days to males, a dose producing an AUC value of more than 25 times the human male AUC.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Sildenafil Tablets, USP are supplied as round shaped, blue colored (mottled), biconvex, uncoated, tablets containing sildenafil citrate equivalent to the nominally indicated amount of sildenafil and debossed on the obverse and reverse sides as follows:
|
25 mg |
50 mg |
100 mg | |
|
Obverse |
407 |
408 |
409 |
|
Reverse |
N |
N |
N |
|
Bottle of 30 |
NDC-70954-407-10 |
NDC-70954-408-10 |
NDC-70954-409-10 |
Recommended Storage: Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
