Elrexfio
These highlights do not include all the information needed to use ELREXFIO safely and effectively. See full prescribing information for ELREXFIO. ELREXFIO (elranatamab-bcmm) injection, for subcutaneous useInitial U.S. Approval: 2023
aa044060-5b4b-4692-bf0f-9a50e140b10e
HUMAN PRESCRIPTION DRUG LABEL
Jul 31, 2025
U.S. Pharmaceuticals
DUNS: 829076905
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
elranatamab-bcmm
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
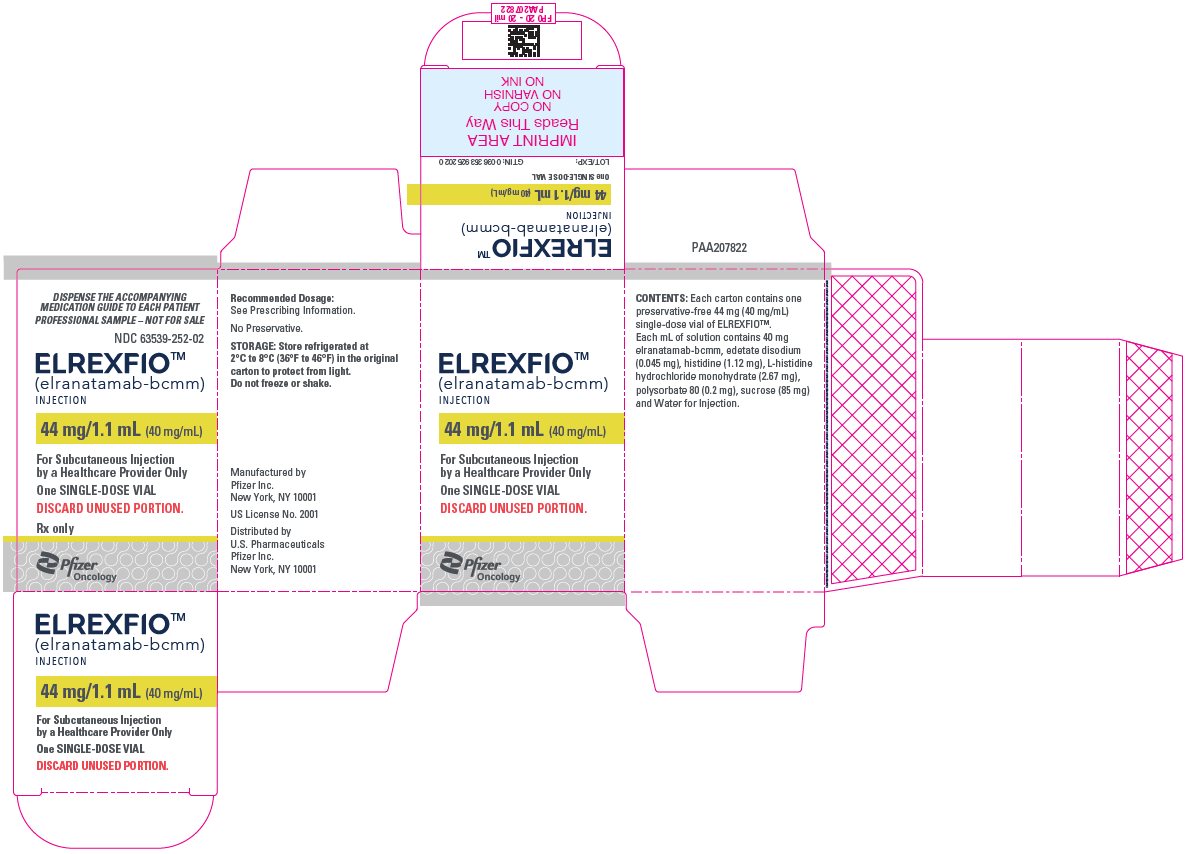
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL – 44 mg/1.1 mL Vial Carton
DISPENSE THE ACCOMPANYING
MEDICATION GUIDE TO EACH PATIENT
PROFESSIONAL SAMPLE – NOT FOR SALE
NDC 63539-252-02
ELREXFIOTM****
****(elranatamab- bcmm)
INJECTION
44 mg/1.1 mL (40 mg/mL)
For Subcutaneous Injection
by a Healthcare Provider Only
One SINGLE-DOSE VIAL
DISCARD UNUSED PORTION.
Rx only
Pfizer
Oncology
Boxed Warning section
WARNING: CYTOKINE RELEASE SYNDROME and NEUROLOGIC TOXICITY including IMMUNE
EFFECTOR CELL-ASSOCIATED NEUROTOXICITY SYNDROME
See full prescribing information for complete boxed warning.
•
**Cytokine Release Syndrome (CRS), including life-threatening or fatal reactions, can occur in patients receiving ELREXFIO. Initiate treatment with ELREXFIO step-up dosing schedule to reduce risk of CRS. Withhold ELREXFIO until CRS resolves or permanently discontinue based on severity. (****2.2****,****2.5****,****5.1****)**
•
**Neurologic toxicity, including Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS), and serious and life-threatening reactions, can occur in patients receiving ELREXFIO. Monitor patients for signs and symptoms of neurologic toxicity, including ICANS, during treatment. Withhold ELREXFIO until the neurologic toxicity resolves or permanently discontinue based on severity. (****2.5****,****5.2****)**
•
**ELREXFIO is available only through a restricted program called the ELREXFIO Risk Evaluation and Mitigation Strategy (REMS). (****5.3****)**
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Cytokine Release Syndrome (CRS)
ELREXFIO can cause CRS, including life-threatening or fatal reactions [see Adverse Reactions (6.1)].
In the clinical trial, CRS occurred in 58% of patients who received ELREXFIO at the recommended dosing schedule [see Dosage and Administration (2.2)], with Grade 1 CRS in 44% of patients, Grade 2 CRS in 14% of patients, and Grade 3 CRS in 0.5% of patients. Recurrent CRS occurred in 13% of patients. Most patients experienced CRS after the first step-up dose (43%) or the second step-up dose (19%), with 7% of patients having CRS after the first treatment dose and 1.6% of patients after a subsequent dose. The median time to onset of CRS was 2 (range: 1 to 9) days after the most recent dose, with a median duration of 2 (range: 1 to 19) days.
Clinical signs and symptoms of CRS may include, but are not limited to, fever, hypoxia, chills, hypotension, tachycardia, headache, and elevated liver enzymes.
Initiate therapy according to the ELREXFIO step-up dosing schedule to reduce risk of CRS and monitor patients following administration of ELREXFIO accordingly [see Dosage and Administration (2.2, 2.5)]. Administer pre- treatment medications prior to each dose in the step-up dosing schedule to reduce risk of CRS [see Dosage and Administration (2.3)].
Counsel patients to seek medical attention should signs or symptoms of CRS occur. At the first sign of CRS, evaluate patients immediately for hospitalization. Manage CRS according to the recommendations and consider further management per current practice guidelines. Withhold or permanently discontinue ELREXFIO based on severity [see Dosage and Administration (2.5)].
ELREXFIO is available only through a restricted program under a REMS [see Warnings and Precautions (5.3)].
5.2 Neurologic Toxicity, Including Immune Effector Cell-Associated
Neurotoxicity Syndrome (ICANS)
ELREXFIO can cause serious or life-threatening neurologic toxicity, including ICANS [see Adverse Reactions (6.1)].
In the clinical trial, neurologic toxicity occurred in 59% of patients who received ELREXFIO at the recommended dosing schedule [see Dosage and Administration (2.2)], with Grade 3 or 4 neurologic toxicity occurring in 7% of patients. Neurologic toxicities included headache (18%), encephalopathy (15%), motor dysfunction (13%), sensory neuropathy (13%), and Guillain-Barré Syndrome (0.5%).
In the clinical trial, ICANS occurred in 3.3% of patients who received ELREXFIO at the recommended dosing schedule [see Dosage and Administration (2.2)]. Most patients had ICANS after the first step-up dose (2.7%), 1 (0.5%) patient had ICANS after the second step-up dose and 1 (0.5%) patient had ICANS after subsequent dose(s). Recurrent ICANS occurred in 1.1% of patients. The median time to onset was 3 (range: 1 to 4) days after the most recent dose, with a median duration of 2 (range: 1 to 18) days. The most frequent clinical manifestations of ICANS included a depressed level of consciousness and Grade 1 or Grade 2 Immune Effector Cell-Associated Encephalopathy (ICE) scores. The onset of ICANS can be concurrent with CRS, following resolution of CRS, or in the absence of CRS.
Counsel patients to seek medical attention should signs or symptoms of neurologic toxicity occur. Monitor patients for signs and symptoms of neurologic toxicities during treatment with ELREXFIO. At the first sign of neurologic toxicity, including ICANS, evaluate and treat patients immediately based on severity. Withhold or permanently discontinue ELREXFIO based on severity per recommendations [see Dosage and Administration (2.5)] and consider further management per current practice guidelines.
Due to the potential for neurologic toxicity including ICANS, patients receiving ELREXFIO are at risk of depressed level of consciousness. Advise patients not to drive or operate heavy or potentially dangerous machinery for 48 hours after completing each of the 2 step-up doses and the first treatment dose within the ELREXFIO step-up dosing schedule and in the event of new onset of any neurological toxicity symptoms until symptoms resolve [see Dosage and Administration (2.2)].
ELREXFIO is available only through a restricted program under a REMS [see Warnings and Precautions (5.3)].
5.3 ELREXFIO REMS
ELREXFIO is available only through a restricted program under a REMS called the ELREXFIO REMS because of the risks of CRS and neurologic toxicity, including ICANS [see Warnings and Precautions (5.1, 5.2)].
Notable requirements of the ELREXFIO REMS include the following:
•
Prescribers must be certified with the program by enrolling and completing training.
•
Prescribers must counsel patients receiving ELREXFIO about the risk of CRS and neurologic toxicity, including ICANS, and provide patients with ELREXFIO Patient Wallet Card.
•
Pharmacies and healthcare settings that dispense ELREXFIO must be certified with the ELREXFIO REMS program and must verify prescribers are certified through the ELREXFIO REMS program.
•
Wholesalers and distributers must only distribute ELREXFIO to certified pharmacies or healthcare settings.
Further information about the ELREXFIO REMS program is available at www.ELREXFIOREMS.com or by telephone at 1‑844‑923‑7845.
5.4 Infections
ELREXFIO can cause severe, life-threatening, or fatal infections. In the clinical trial, in patients who received ELREXFIO according to the recommended dosing schedule, serious infections, including opportunistic infections, occurred in 42% of patients, with Grade 3 or 4 infections in 31%, and fatal infections in 7%. The most common serious infections reported (≥5%) were pneumonia and sepsis [see Adverse Reactions (6.1)].
Do not initiate treatment with ELREXFIO in patients with active infections. Monitor patients for signs and symptoms of infection prior to and during treatment with ELREXFIO and treat appropriately. Withhold or permanently discontinue ELREXFIO based on severity [see Dosage and Administration (2.5)]. Administer prophylactic antimicrobial and anti-viral medications according to current practice guidelines. Consider treatment with subcutaneous or intravenous immunoglobulin (IVIG) as appropriate.
5.5 Neutropenia
ELREXFIO can cause neutropenia and febrile neutropenia. In patients who received ELREXFIO at the recommended dose in the clinical trial, decreased neutrophils occurred in 62% of patients, with Grade 3 or 4 decreased neutrophils in 51%. Febrile neutropenia occurred in 2.2% of patients [see Adverse Reactions (6.1)].
Monitor complete blood cell counts at baseline and periodically during treatment. Provide supportive care according to current practice guidelines. Monitor patients with neutropenia for signs of infection. Withhold ELREXFIO based on severity [see Dosage and Administration (2.5)].
5.6 Hepatotoxicity
ELREXFIO can cause hepatotoxicity. In the clinical trial, elevated ALT occurred in 36% of patients, with Grade 3 or 4 ALT elevation occurring in 3.8%; elevated AST occurred in 40% of patients, with Grade 3 or 4 AST elevation occurring in 6%. Grade 3 or 4 total bilirubin elevations occurred in 0.5% of patients [see Adverse Reactions (6.1)]. Liver enzyme elevation can occur with or without concurrent CRS.
Monitor liver enzymes and bilirubin at baseline and during treatment as clinically indicated. Withhold ELREXFIO or consider permanent discontinuation of ELREXFIO based on severity [see Dosage and Administration (2.5)].
5.7 Embryo-Fetal Toxicity
Based on its mechanism of action, ELREXFIO may cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to the fetus. Advise females of reproductive potential to use effective contraception during treatment with ELREXFIO and for 4 months after the last dose [see Use in Specific Populations (8.1, 8.3)].
•
Infections: Can cause severe, life-threatening, or fatal infections. Monitor patients for signs and symptoms of infection and treat appropriately. Do not initiate treatment in patients with active infections. (5.4)
•
Neutropenia: Monitor complete blood cell counts at baseline and periodically during treatment. (5.5)
•
Hepatotoxicity: Can cause elevated ALT, AST, and bilirubin. Monitor liver enzymes and bilirubin at baseline and during treatment as clinically indicated. (5.6)
•
Embryo-Fetal Toxicity: May cause fetal harm. Advise females of reproductive potential of the potential risk to the fetus and to use effective contraception. (5.7, 8.1. 8.3)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following adverse reactions are discussed elsewhere in labeling:
•
Cytokine Release Syndrome [see Warnings and Precautions (5.1)].
•
Neurologic Toxicity, Including ICANS [see Warnings and Precautions (5.2)].
•
Infections [see Warnings and Precautions (5.4)].
•
Neutropenia [see Warnings and Precautions (5.5)].
•
Hepatotoxicity [see Warnings and Precautions (5.6)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Relapsed/Refractory Multiple Myeloma
MagnetisMM-3
The safety of ELREXFIO was evaluated in MagnetisMM-3 [see Clinical Studies (14)]. The safety population described (n = 183) includes patients who received the recommended dosage regimen of 12 mg subcutaneously on Day 1, 32 mg on Day 4, and 76 mg once weekly starting on Day 8. Among patients who received ELREXFIO, 42% were exposed for 6 months or longer and 9% were exposed for one year or longer.
The median age of patients who received ELREXFIO was 68 years (range: 36 to 88 years); 48% were female; 61% were White, 10% were Hispanic/Latino, 9% were Asian, and 6% were Black or African American.
Serious adverse reactions occurred in 68% of patients who received ELREXFIO at the recommended dosing schedule. Serious adverse reactions in >2% of patients included pneumonia (25%), sepsis (13%), CRS (13%), upper respiratory tract infection (4.4%), acute kidney injury (3.8%), urinary tract infection (3.3%), COVID-19 (3.3%), encephalopathy (3.3%), pyrexia (2.2%), and febrile neutropenia (2.2%). Fatal adverse reactions occurred in 10% of patients including pneumonia (3.3%), sepsis (2.7%), acute respiratory distress syndrome (0.5%), cardio-respiratory arrest (0.5%), cardiogenic shock (0.5%), cardiopulmonary failure (0.5%), COVID-19 (0.5%), failure to thrive (0.5%), and pulmonary embolism (0.5%).
Permanent discontinuations of ELREXFIO due to an adverse reaction occurred in 17% of patients. Adverse reactions which resulted in permanent discontinuation of ELREXFIO in >2% of patients included septic shock (2.2%).
Dosage interruptions of ELREXFIO due to an adverse reaction occurred in 73% of patients. Adverse reactions which resulted in dose interruptions of ELREXFIO in >5% of patients included neutropenia, pneumonia, COVID-19, upper respiratory tract infection, thrombocytopenia, and anemia.
The most common adverse reactions (≥20%) were CRS, fatigue, injection site reaction, diarrhea, upper respiratory tract infection, musculoskeletal pain, pneumonia, decreased appetite, rash, cough, nausea, and pyrexia. The most common Grade 3 to 4 laboratory abnormalities (≥30%) were decreased lymphocytes, decreased neutrophils, decreased hemoglobin, decreased white blood cells, and decreased platelets.
Table 8 summarizes adverse reactions in MagnetisMM-3.
Table 8. Adverse Reactions (≥10%) in Patients with Relapsed or Refractory Multiple Myeloma Who Received ELREXFIO in MagnetisMM-3|
Adverse reactions were graded based on CTCAE Version 5.0, with the exception of CRS, which was graded based on the ASTCT 2019 criteria. | ||
Þ ß | ||
|
System Organ Class Preferred Term |
ELREXFIO N = 183 | |
|
All Grades (%) |
Grade 3 or 4 (%) | |
|
Immune system disorders | ||
|
Cytokine release syndrome |
58 |
0.5* |
|
Hypogammaglobulinemia† |
13 |
2.2* |
|
General disorders and site administration conditions | ||
|
Fatigue† |
43 |
6* |
|
Injection site reaction† |
37 |
0 |
|
Pyrexia |
21 |
2.7* |
|
Edema† |
18 |
1.1* |
|
Gastrointestinal disorders | ||
|
Diarrhea |
36 |
1.1* |
|
Nausea |
21 |
0 |
|
Constipation |
14 |
0 |
|
Vomiting |
14 |
0 |
|
Infections | ||
|
Upper respiratory tract infection† |
36 |
4.9 |
|
Pneumonia‡ |
32 |
19 |
|
Sepsis§ |
15 |
11 |
|
Urinary tract infection† |
12 |
4.4* |
|
Musculoskeletal and connective tissue disorders | ||
|
Musculoskeletal pain† |
34 |
2.7* |
|
Metabolism and nutrition disorders | ||
|
Decreased appetite |
26 |
1.1* |
|
Skin and Subcutaneous Tissue disorders | ||
|
Rash¶ |
26 |
0 |
|
Dry skin |
14 |
0 |
|
Skin exfoliation† |
10 |
0 |
|
Respiratory, thoracic and mediastinal disorders | ||
|
Cough† |
24 |
0 |
|
Dyspnea† |
15 |
3.3* |
|
Nervous system disorders | ||
|
Headache |
18 |
0 |
|
Encephalopathy# |
14 |
2.2 |
|
Sensory neuropathyÞ |
13 |
0.5* |
|
Motor dysfunctionß |
14 |
1.1* |
|
Cardiac disorders | ||
|
Cardiac arrhythmia† |
16 |
1.6 |
|
Vascular disorders | ||
|
Hemorrhage† |
13 |
1.6 |
|
Psychiatric disorders | ||
|
Insomnia |
13 |
0 |
|
Injury, poisoning and procedural complications | ||
|
Fall |
10 |
0.5* |
Clinically relevant adverse reactions in <10% of patients who received ELREXFIO included ICANS, febrile neutropenia, Guillain-Barré syndrome, abdominal pain, acute kidney injury, COVID-19, cardiac failure, congestion, thrombosis and cytomegalovirus infection.
Table 9 summarizes laboratory abnormalities in MagnetisMM-3.
Table 9. Select Laboratory Abnormalities (≥30%) That Worsened from Baseline in Patients with Relapsed or Refractory Multiple Myeloma Who Received ELREXFIO in MagnetisMM-3*
| ||
|
Laboratory Abnormality |
ELREXFIO† | |
|
All Grades (%) |
Grade 3 or 4 (%) | |
|
Hematology | ||
|
Lymphocyte count decreased |
91 |
84 |
|
White blood cell decreased |
69 |
40 |
|
Hemoglobin decreased |
68 |
43 |
|
Neutrophil count decreased |
62 |
51 |
|
Platelet count decreased |
61 |
32 |
|
Chemistry | ||
|
Albumin decreased |
55 |
6 |
|
AST increase |
40 |
6 |
|
Creatinine increased |
38 |
3.3 |
|
Potassium decreased |
36 |
8 |
|
ALT increase |
36 |
3.8 |
|
Alkaline phosphatase increased |
34 |
1.1 |
|
Creatinine clearance decreased |
32 |
10 |
Most common adverse reactions (incidence ≥20%) are CRS, fatigue, injection site reaction, diarrhea, upper respiratory tract infection, musculoskeletal pain, pneumonia, decreased appetite, rash, cough, nausea, and pyrexia.
The most common Grade 3 to 4 laboratory abnormalities (≥30%) are decreased lymphocytes, decreased neutrophils, decreased hemoglobin, decreased white blood cells, and decreased platelets. (6.1)
**To report SUSPECTED ADVERSE REACTIONS, contactPfizer Inc. at 1-800-438-1985 or FDA at 1-800-FDA-1088 or **www.fda.gov/medwatch.
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
For certain CYP substrates, minimal changes in the concentration may lead to serious adverse reactions. Monitor for toxicity or drug concentrations of such CYP substrates when co-administered with ELREXFIO.
ELREXFIO causes release of cytokines [see Clinical Pharmacology (12.2)] that may suppress activity of cytochrome P450 (CYP) enzymes, resulting in increased exposure of CYP substrates. Increased exposure of CYP substrates is more likely to occur after the first dose of ELREXFIO Day 1 and up to 14 days after the 32 mg dose on Day 4 and during and after CRS [see Warnings and Precautions (5.1)].
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Elranatamab-bcmm is a bispecific B-cell maturation antigen (BCMA)-directed T-cell engaging antibody that binds BCMA on plasma cells, plasmablasts, and multiple myeloma cells and CD3 on T-cells leading to cytolysis of the BCMA- expressing cells. Elranatamab-bcmm activated T-cells, caused proinflammatory cytokine release, and resulted in multiple myeloma cell lysis.
12.2 Pharmacodynamics
Cytokine Concentrations
Transient elevation of circulating cytokines IL-2, IL-6, IL-8, IL-10, TNF-α, and IFN-γ was observed at dosage levels of 30 µg/kg (0.03 times the approved recommended dosage) and above. After administration of the approved recommended dosage of ELREXFIO, the highest elevation of cytokines was generally observed within 72 hours after first elranatamab-bcmm dose at 12 mg on Day 1, and generally returned to baseline prior to the administration of the first full dose 76 mg on Day 8.
12.3 Pharmacokinetics
Pharmacokinetic parameters are presented as geometric mean (coefficient of variation [CV]%) and are based upon subcutaneously administered unless otherwise specified.
Elranatamab-bcmm exhibits dose proportional pharmacokinetics over dose range from 6 to 76 mg (0.079 to 1 times the approved recommended dosage). Elranatamab-bcmm maximum concentration [33.0 mcg/mL (46%)] is achieved at the end of weekly dosing regimen (i.e., at week 24 of 76 mg weekly dosing). Pharmacokinetic exposures are summarized for the recommended dosage of ELREXFIO in Table 10.
Table 10. Pharmacokinetic Parameters of Elranatamab-bcmm in Subjects with Relapsed or Refractory Multiple Myeloma
| |||
|
Timepoint |
Parameters | ||
|
C****avg (mcg/mL) |
C****max (mcg/mL) |
C****trough (mcg/mL) | |
|
First full 76 mg dose |
3.1 (94%) |
3.8 (94%) |
3.3 (102%) |
|
End of weekly dose (week 24)* |
32.0 (46%) |
33.0 (46%) |
30.5 (48%) |
|
Steady state (biweekly dosing)*† |
17.7 (53%) |
19.5 (51%) |
15.1 (60%) |
|
Steady state (every 4 weeks dosing)*‡ |
8.8 (58%) |
11.5 (54%) |
5.9 (78%) |
Absorption
The mean bioavailability of elranatamab-bcmm was 56.2% when administered subcutaneously. The median (min, max) Tmax after elranatamab SC administration was 7 (3 to 7) days.
Distribution
The steady state volume of distribution of elranatamab-bcmm was 7.76 L (33%).
Elimination
The half-life of elranatamab-bcmm is 22 (64%) days at the 76 mg dosage, with clearance of 0.324 L/day (100%) following 24 weeks dosing.
Metabolism
Elranatamab-bcmm is expected to be metabolized into small peptides by catabolic pathways.
Specific Populations
No clinically significant differences in the pharmacokinetics of elranatamab- bcmm were observed based on age (36 to 89 years), sex, race (White, Asian, or Black), body weight (37 to 160 kg), mild or moderate renal impairment (estimated glomerular filtration rate [eGFR] by Modification of Diet in Renal Disease [MDRD] method: 30 to 89 mL/min), or mild hepatic impairment (total bilirubin 1 to ≤1.5 x ULN or any AST greater than ULN).
The effects of severe renal impairment (eGFR 15 to 29 mL/min), end-stage renal disease (eGFR <15 mL/min), or moderate to severe hepatic impairment (total bilirubin >1.5 times ULN and any AST) on the PK of elranatamab-bcmm are unknown.
12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies, including those of elranatamab-bcmm or of other elranatamab products.
In the MagnetisMM-3 study, of the 168 participant who received recommended step-up and full dosage of ELREXFIO for up to 36 months and are evaluable for presence of ADA against elranatamab-bcmm, 9.5% (16/168) of patients tested positive for anti-elranatamab-bcmm-antibodies. Among the 16 patients who tested positive for ADAs, 56% (9/16) tested positive for neutralizing antibodies against elranatamab-bcmm. The effect of these antibodies on the pharmacokinetics, pharmacodynamics, safety, and/or effectiveness of ELREXFIO products is unknown.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Cytokine Release Syndrome (CRS)
Discuss the signs and symptoms associated with CRS, including fever, hypoxia, chills, hypotension, tachycardia, and elevated liver enzymes. Advise patients to immediately contact their healthcare provider if they experience any signs or symptoms of CRS. Advise patients that they will be hospitalized for 48 hours after administration of the first step-up dose, and for 24 hours after administration of the second step-up dose [see Dosage and Administration (2.5), Warnings and Precautions (5.1)].
Neurologic Toxicity, Including Immune Effector Cell-associated Neurotoxicity Syndrome (ICANS)
Discuss the signs and symptoms associated with neurologic toxicity, including ICANS, including headache, encephalopathy, motor dysfunction, sensory neuropathy, and Guillain-Barré Syndrome. Advise patients to immediately contact their healthcare provider if they experience any signs or symptoms of neurologic toxicity. Advise patients to refrain from driving or operating heavy or potentially dangerous machinery for 48 hours after completing each of the 2 step-up doses and the first treatment dose within the ELREXFIO step-up dosing schedule and in the event of new onset of any neurological toxicity symptoms until symptoms resolve [see Dosage and Administration (2.5), Warnings and Precautions (5.2)].
ELREXFIO REMS
ELREXFIO is available only through a restricted program called ELREXFIO REMS. Inform patients that they will be given an ELREXFIO Patient Wallet Card that they should carry with them at all times and show to all of their healthcare providers. This card describes signs and symptoms of CRS and neurologic toxicity, including ICANS which, if experienced, should prompt the patient to immediately seek medical attention [see Warnings and Precautions (5.3)].
Infections
Discuss the signs and symptoms of infection [see Dosage and Administration (2.5), Warnings and Precautions (5.4)].
Neutropenia
Discuss the signs and symptoms associated with neutropenia and febrile neutropenia [see Dosage and Administration (2.5), Warnings and Precautions (5.5)].
Hepatotoxicity
Advise patients that liver enzyme elevations may occur and that they should report symptoms that may indicate liver toxicity, including fatigue, anorexia, right upper abdominal discomfort, dark urine, or jaundice [see Warnings and Precautions (5.6)].
Embryo-Fetal Toxicity
Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to inform their healthcare provider if they are pregnant or become pregnant. Advise females of reproductive potential to use effective contraception during treatment with ELREXFIO and for 4 months after the last dose [see Warnings and Precautions (5.7), Use in Specific Populations (8.1, 8.3)].
Lactation
Advise women not to breastfeed during treatment with ELREXFIO and for 4 months after the last dose [see Use in Specific Populations (8.2)].
Manufactured by:
Pfizer Inc.
NY, NY 10001
US License No. 2001

LAB-1518-3.0
SPL MEDGUIDE SECTION
|
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: JUL 2025 | |||
|
MEDICATION GUIDE ELREXFIOTM (el-reks-fe-o) (elranatamab-bcmm) | |||
|
What is the most important information I should know about ELREXFIO? ELREXFIO may cause serious side effects, including: • | |||
|
o o o o o |
o o | ||
o ▪ ▪ ▪ o o o • | |||
|
o o o |
o o o o | ||
|
• Your healthcare provider will monitor you for signs and symptoms of CRS and neurologic problems during treatment with ELREXFIO, as well as other side effects, and will treat you if needed. Your healthcare provider may temporarily stop or completely stop your treatment with ELREXFIO if you develop CRS, neurologic problems, or any other side effects that are severe. If you have any questions about ELREXFIO, ask your healthcare provider. See “What are possible side effects of ELREXFIO?” for more information about side effects. | |||
|
What is ELREXFIO? ELREXFIO is a prescription medicine used to treat adults with multiple myeloma who: • • It is not known if ELREXFIO is safe and effective in children. | |||
|
Before receiving ELREXFIO, tell your healthcare provider about all of your medical conditions, including if you: • • Females who are able to become pregnant: o o o • Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. | |||
|
How will I receive ELREXFIO? • • • • • • If you miss any appointments, call your healthcare provider as soon as possible to reschedule your appointment. It is important for you to be monitored closely for side effects during treatment with ELREXFIO. | |||
|
What should I avoid while receiving ELREXFIO? Do not drive, operate heavy or potentially dangerous machinery, or do other dangerous activities during treatment with ELREXFIO: • • See “What is the most important information I should know about ELREXFIO?” for more information about signs and symptoms of neurologic problems. | |||
|
What are the possible side effects of ELREXFIO? ELREXFIO may cause serious side effects, including: • • o o | |||
|
▪ ▪ ▪ ▪ |
▪ ▪ ▪ ▪ | ||
|
• • | |||
|
o o o |
o o | ||
|
Your healthcare provider will check your blood and monitor you for signs and symptoms of these serious side effects before you start and during treatment with ELREXFIO and may temporarily or completely stop treatment with ELREXFIO if you develop certain side effects. The most common side effects of ELREXFIO include: | |||
|
• • • • |
• • • • • | ||
|
The most common severe abnormal blood test results with ELREXFIO include decreased white blood cells, red blood cells, and platelets. These are not all of the possible side effects of ELREXFIO. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. | |||
|
General information about the safe and effective use of ELREXFIO. Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. You can ask your pharmacist or healthcare provider for more information about ELREXFIO that is written for health professionals. | |||
|
What are the ingredients in ELREXFIO? **Active ingredient:**elranatamab-bcmm Inactive ingredients: edetate disodium, histidine, L-histidine hydrochloride monohydrate, polysorbate 80, sucrose, and Water for Injection Manufactured by: Pfizer Inc., NY, NY 10001 US License No. 2001
LAB-1551-2.0 For more information on ELREXFIO, go to www.ELREXFIO.com For more information on Pfizer, go to www.Pfizer.com or call 1-800-438-1985 |
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Relapsed or Refractory Multiple Myeloma
The efficacy of ELREXFIO monotherapy was evaluated in patients with relapsed or refractory multiple myeloma in an open-label, single arm, multi-center study (MagnetisMM-3, NCT04649359). The study included patients who were refractory to at least one proteasome inhibitor (PI), one immunomodulatory agent (IMiD), and one anti-CD38 monoclonal antibody. MagnetisMM-3 included 123 patients naïve to prior BCMA-directed therapy (pivotal Cohort A) and 64 patients with prior BCMA-directed antibody drug conjugate (ADC) or chimeric antigen receptor (CAR) T-cell therapy (supportive Cohort B). Patients had measurable disease by International Myeloma Working Group (IMWG) criteria at enrollment. The study included patients with an Eastern Cooperative Oncology Group (ECOG) score of ≤2, adequate baseline bone marrow (absolute neutrophil count ≥1.0 x 109/L, platelet count ≥25 x 109/L, hemoglobin level ≥8 g/dL), renal (CrCL ≥ 30 mL/min), and hepatic (AST and ALT ≤2.5 x ULN, total bilirubin ≤2 x ULN) function, and left-ventricular ejection fraction ≥40%. Patients with a stem cell transplant within 12 weeks prior to enrollment and active infections were excluded from the study.
Eligible patients received subcutaneous administration of ELREXFIO at step-up doses of 12 mg on Day 1 and 32 mg on Day 4 of treatment, followed by the first treatment dose of ELREXFIO (76 mg) on Day 8 of treatment. Thereafter, patients received 76 mg once weekly. After 24 weeks, in patients who achieved an IMWG response category of partial response or better with responses persisting for at least 2 months, the dose interval was changed from every week to every 2 weeks.
The 123 patients enrolled in pivotal Cohort A had received a median of 5 prior lines of therapy (range: 2 to 22). Ninety-seven patients who were not exposed to prior BCMA-directed therapy and received at least four prior lines of therapy comprised the efficacy population. Among the 97 patients in the efficacy population, the median age was 69 (range: 46 to 89) years with 18.6% of patients ≥75 years of age. Forty percent were female; 59.8% were White, 13.4% were Asian, 7.2% were Hispanic/Latino, 5.2% were Black or African American. Disease stage (R-ISS) at study entry was 20.6% in Stage I, 53.6% in Stage II, and 17.5% in Stage III. The median time since initial diagnosis of multiple myeloma to enrollment was 79.6 (range: 16 to 228) months. 96.9% were triple-class refractory, and 94.8% were refractory to their last line of therapy. 69.1% received prior autologous stem cell transplantation, and 7.2% received prior allogenic stem cell transplantation. High-risk cytogenetics [t(4;14), t(14;16), or del(17p)] were present in 22.7% of patients. 34.0% of patients had extramedullary disease at baseline by BICR.
Efficacy was based on response rate and duration of response (DOR), as assessed by BICR based on IMWG criteria. Efficacy results from BCMA-directed therapy naïve patients are shown in Table 11.
The median (range) time to first response (TTR) was 1.22 (0.9 to 6.5) months. With a median follow-up of 11.1 months (95% CI: 10.6, 12.0) among responders, the DOR rate at 6 months was 90.4% (95% CI: 78.4%, 95.9%) and at 9 months was 82.3% (95% CI: 67.1%, 90.9%).
Table 11. Efficacy Results from BCMA-directed Therapy Naïve Patients|
Abbreviations: CI = Confidence interval; NR = Not reached; NE = Not estimable. | |
| |
|
N = 97 | |
|
Objective Response Rate (ORR: sCR+CR+VGPR+PR), n (%) (95% CI) |
56 (57.7%) (47.3, 67.7) |
|
Complete response (CR) or better* |
25 (25.8%) |
|
Very good partial response (VGPR) |
25 (25.8%) |
|
Partial response (PR) |
6 (6.2%) |
|
Duration of Response (DOR) (months) Median (95% CI) |
NR (12.0, NE) |
Among the 64 patients enrolled in Cohort B who previously received a PI, an IMiD, an anti-CD38 monoclonal antibody, and a BCMA-directed therapy, 63 patients received at least four prior lines of therapy. Patients had received a median of 8 prior lines of therapy (range: 4 to 19); 73% and 32% received prior BCMA-directed ADC and CAR T-cell therapy, respectively.
Confirmed ORR by BICR was 33.3% (95% CI: 22.0, 46.3). After a median (95% CI) follow-up of 10.2 (9.9, 11) months among responders, median DOR was not reached (95% CI: NE, NE) and the DOR rate at 9 months was 84.3% (95% CI: 58.7, 94.7).
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosing Information
Administer ELREXFIO subcutaneously according to the step-up dosing schedule to reduce the incidence and severity of cytokine release syndrome (CRS).
Administer pre-treatment medications prior to each dose in the ELREXFIO step- up dosing schedule, which includes step-up dose 1, step-up dose 2, and the first treatment dose as recommended [see Dosage and Administration (2.2, 2.3)].
ELREXFIO should only be administered by a qualified healthcare professional with appropriate medical support to manage severe reactions such as CRS and neurologic toxicity, including ICANS [see Warnings and Precautions (5.1, 5.2)].
Due to the risk of CRS, patients should be hospitalized for 48 hours after administration of the first step-up dose, and for 24 hours after administration of the second step-up dose.
2.2 Recommended Dosage
For subcutaneous injection only.
The recommended dosing schedule for ELREXFIO is provided in Table 1. The recommended dosages of ELREXFIO subcutaneous injection are: step-up dose 1 of 12 mg on Day 1, step-up dose 2 of 32 mg on Day 4, followed by the first treatment dose of 76 mg on Day 8, and then 76 mg weekly thereafter through week 24.
For patients who have received at least 24 weeks of treatment with ELREXFIO and have achieved a response [partial response (PR) or better] and maintained this response for at least 2 months, the dose interval should transition to an every two-week schedule. For patients who have received at least 24 weeks of treatment with ELREXFIO at the every two-week dosing schedule and have maintained the response, the dose interval should transition to an every four- week schedule.
Continue treatment with ELREXFIO until disease progression or unacceptable toxicity.
Administer pre-treatment medications prior to each dose in the ELREXFIO step- up dosing schedule, which includes step-up dose 1, step-up dose 2, and the first treatment dose as recommended [see Dosage and Administration (2.3)].
Table 1. ELREXFIO Dosing Schedule|
Note: See Table 2 for recommendations on restarting ELREXFIO after dose delays. | |||
| |||
|
Dosing Schedule |
Day |
ELREXFIO Dose | |
|
Step-up Dosing Schedule |
Day 1* |
Step-up dose 1 |
12 mg |
|
Day 4*† |
Step-up dose 2 |
32 mg | |
|
Day 8*‡ |
First treatment dose |
76 mg | |
|
Weekly Dosing Schedule |
One week after first treatment dose and weekly thereafter§ through week 24 |
Subsequent treatment doses |
76 mg |
|
Biweekly (Every 2 Week) Dosing Schedule *Responders only week 25 onward |
Week 25 and every 2 weeks thereafter§ through week 48 |
Subsequent treatment doses |
76 mg |
|
Every 4 Week Dosing Schedule *In patients who have maintained the response following 24 weeks of treatment at the biweekly dosing schedule |
Week 49 and every 4 weeks thereafter§ |
Subsequent treatment doses |
76 mg |
2.3 Recommended Pre-treatment Medications
Administer the following pre-treatment medications approximately 1 hour before the first three doses of ELREXFIO in the step-up dosing schedule, which includes step-up dose 1, step-up dose 2, and the first treatment dose as described in Table 1 to reduce the risk of CRS [see Warnings and Precautions (5.1)]:
•
acetaminophen (or equivalent) 650 mg orally
•
dexamethasone (or equivalent) 20 mg orally or intravenously
•
diphenhydramine (or equivalent) 25 mg orally
2.4 Restarting ELREXFIO After Dosage Delay
If a dose of ELREXFIO is delayed, restart therapy based on the recommendations listed in Table 2 and resume the dosing schedule accordingly [see Dosage and Administration (2.2)]. Administer pre-treatment medications as indicated in Table 2.
Table 2. Recommendation for Restarting Therapy with ELREXFIO After Dosage Delay
| ||
|
Last Dose Administered |
Time Since the Last Dose Administered |
Action for Next Dose |
|
Step-up dose 1 (12 mg) |
2 weeks or less (≤14 days) |
Restart ELREXFIO at step-up dose 2 (32 mg).* If tolerated, increase to 76 mg 4 days later. |
|
Greater than 2 weeks (>14 days) |
Restart ELREXFIO step-up dosing schedule at step-up dose 1 (12 mg).* | |
|
Step-up dose 2 (32 mg) |
2 weeks or less (≤14 days) |
Restart ELREXFIO at 76 mg.* |
|
Greater than 2 weeks to less than or equal to 4 weeks (15 days to ≤28 days) |
Restart ELREXFIO at step-up dose 2 (32 mg).* If tolerated, increase to 76 mg 1 week later. | |
|
Greater than 4 weeks (>28 days) |
Restart ELREXFIO step-up dosing schedule at step-up dose 1 (12 mg).* | |
|
Any weekly treatment dose (76 mg) |
8 weeks or less (≤56 days) |
Restart ELREXFIO at 76 mg. |
|
Greater than 8 weeks to less or equal to 12 weeks (57 days to ≤84 days)† |
Restart ELREXFIO at step-up dose 2 (32 mg).*If tolerated, increase to 76 mg 1 week later. | |
|
Greater than 12 weeks (>84 days)† |
Restart ELREXFIO step-up dosing schedule at step-up dose 1 (12 mg).* | |
|
Any biweekly or every-4-week treatment dose (76 mg) |
12 weeks or less (≤84 days) |
Restart ELREXFIO at 76 mg. |
|
Greater than 12 weeks (>84 days)† |
Restart ELREXFIO step-up dosing schedule at step-up dose 1 (12 mg).* |
2.5 Dosage Modifications for Adverse Reactions
Dosage reductions of ELREXFIO are not recommended.
Dosage delays may be required to manage toxicities related to ELREXFIO [see Warnings and Precautions (5)]. Recommendations on restarting ELREXFIO after a dose delay are provided in Table 2.
See Table 3 and Table 4 for recommended actions for adverse reactions of CRS and ICANS, respectively. See Table 5 for recommended actions for neurologic toxicity excluding ICANS and Table 6 for recommended actions for other adverse reactions following administration of ELREXFIO. Consider further management per current practice guidelines.
Management of CRS, Neurologic Toxicity Including ICANS
Cytokine Release Syndrome (CRS)
Management recommendations for CRS are summarized in Table 3.
Identify CRS based on clinical presentation [see Warnings and Precautions (5.1)]. Evaluate and treat other causes of fever, hypoxia, and hypotension.
If CRS is suspected, withhold ELREXFIO until CRS resolves. Manage CRS according to the recommendations in Table 3 and consider further management per current practice guidelines. Administer supportive therapy for CRS, which may include intensive care for severe or life-threatening CRS. Consider laboratory testing to monitor for disseminated intravascular coagulation (DIC), hematology parameters, as well as pulmonary, cardiac, renal, and hepatic function.
Table 3. Recommendations for Management of CRS
| ||
|
Grade* |
Presenting Symptoms |
Actions |
|
Grade 1 |
Temperature ≥100.4 °F (38 °C)† |
• • |
|
Grade 2 |
Temperature ≥100.4 °F (38 °C) with either: • • |
• • • |
|
Grade 3 (First occurrence) |
Temperature ≥100.4 °F (38 °C) with either: • • |
• • • • |
|
Grade 3 (Recurrent) |
Temperature ≥100.4 °F (38 °C) with either: • • |
• • |
|
Grade 4 |
Temperature ≥100.4 °F (38 °C) with either: • • |
• • |
Neurologic Toxicity Including ICANS
Management recommendations for ICANS and neurologic toxicity are summarized in Table 4 and Table 5.
At the first sign of neurologic toxicity, including ICANS, withhold ELREXFIO and consider neurology evaluation. Rule out other causes of neurologic symptoms. Provide supportive therapy, which may include intensive care, for severe or life-threatening neurologic toxicities, including ICANS [see Warnings and Precautions (5.2)]. Manage ICANS according to the recommendations in Table 4 and consider further management per current practice guidelines.
Table 4. Recommendations for Management of ICANS
| ||
|
Grade* |
Presenting Symptoms† |
Actions |
|
Grade 1 |
ICE score 7-9‡ Or depressed level of consciousness§: awakens spontaneously. |
• • • |
|
Grade 2 |
ICE score 3-6‡ Or depressed level of consciousness§: awakens to voice. |
• • • • • |
|
Grade 3 (First occurrence) |
ICE score 0-2‡ or depressed level of consciousness§: awakens only to tactile stimulus, or seizures§, either: • • or raised intracranial pressure: focal/local edema on neuroimaging§ |
• • • • • • |
|
Grade 3 (recurrent) |
ICE score 0-2‡ or depressed level of consciousness§: awakens only to tactile stimulus, or seizures§, either: • • or raised intracranial pressure: focal/local edema on neuroimaging§ |
• • • • • |
|
Grade 4 |
ICE score 0‡ Or, depressed level of consciousness§ either: • • or seizures§, either: • • or motor findings§: • or raised intracranial pressure/cerebral edema§, with signs/symptoms such as: • • • • • |
• • • • • • |
|
Adverse Reaction |
Severity |
Actions |
|
Neurologic Toxicity (excluding ICANS) |
Grade 1 |
• |
|
Grade 2 Grade 3 (First occurrence) |
• • | |
|
Grade 3 (Recurrent) Grade 4 |
• • |
| ||
|
Adverse Reactions |
Severity |
Actions |
|
Hematologic Adverse Reactions [see Warnings and Precautions (5.5)] |
Absolute neutrophil count less than 0.5 x 109/L |
• |
|
Febrile neutropenia |
• | |
|
Hemoglobin less than 8 g/dL |
• | |
|
Platelet count less than 25,000/mcL Platelet count between 25,000/mcL and 50,000/mcL with bleeding |
• | |
|
Infections and Other Non-hematologic Adverse Reactions† [see Warnings and Precautions (5.4, 5.6) and Adverse Reactions (6.1)] |
Grade 3 |
• |
|
Grade 4 |
• • |
2.6 Preparation and Administration Instructions
ELREXFIO is intended for subcutaneous use by a healthcare provider only.
ELREXFIO should be administered by a healthcare provider with adequate medical personnel and appropriate medical equipment to manage severe reactions, including CRS and neurologic toxicity, including ICANS [see Warnings and Precautions (5.1, 5.2)].
ELREXFIO 76 mg/1.9 mL (40 mg/mL) vial and 44 mg/1.1 mL (40 mg/mL) vial are supplied as ready-to-use solution that do not need dilution prior to administration.
ELREXFIO is a clear to slightly opalescent, and colorless to pale brown liquid solution. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not administer if solution is discolored or contains particulate matter.
Use aseptic technique to prepare and administer ELREXFIO.
Preparation
ELREXFIO vials are for one-time use in a single patient and do not contain any preservatives.
Prepare ELREXFIO following the instructions below (see Table 7) depending on the required dose.
Table 7. Injection Volumes|
Total Dose (mg) |
Volume of Injection |
|
12 mg |
0.3 mL |
|
32 mg |
0.8 mL |
|
76 mg |
1.9 mL |
Remove the appropriate strength ELREXFIO vial from refrigerated storage [2 °C to 8 °C (36 °F to 46 °F)]. Once removed from refrigerated storage, equilibrate ELREXFIO to ambient temperature [15 °C to 30 °C (59 °F to 86 °F)]. Do not warm ELREXFIO in any other way.
Withdraw the required injection volume of ELREXFIO from the vial into an appropriately sized syringe with stainless steel injection needles (30G or wider) and polypropylene or polycarbonate syringe material. Discard unused portion.
Administration
Inject the required volume of ELREXFIO into the subcutaneous tissue of the abdomen (preferred injection site). Alternatively, ELREXFIO may be injected into the subcutaneous tissue at other sites (e.g., thigh).
Do not inject into tattoos or scars or areas where the skin is red, bruised, tender, hard or not intact.
Storage of Prepared Syringe
If the prepared dosing syringe is not used immediately, the syringe may be stored refrigerated between 2 °C to 8 °C (36 °F to 46 °F) for a maximum of 72 hours or between 8 °C to 25 °C (46 °F to 77 °F) for a maximum of 24 hours. Once removed from refrigerated storage, the prepared syringe must be used or discarded.
| |||
|
ELREXFIO Dosing Schedule (2.2) | |||
|
Dosing Schedule |
Day |
ELREXFIO Dose | |
|
Step-up Dosing Schedule |
Day 1 |
Step-up dose 1 |
12 mg |
|
Day 4 |
Step-up dose 2 |
32 mg | |
|
Day 8 |
First treatment dose |
76 mg | |
|
Weekly Dosing Schedule |
One week after first treatment dose and weekly thereafter through week 24 |
Subsequent treatment doses |
76 mg |
|
Biweekly (Every 2 Week) Dosing Schedule* |
Week 25 and every 2 weeks thereafter through week 48 |
Subsequent treatment doses |
76 mg |
|
Every 4 Week Dosing Schedule† |
Week 49 and every 4 weeks thereafter |
Subsequent treatment doses |
76 mg |
•
Patients should be hospitalized for 48 hours after administration of the first step-up dose, and for 24 hours after administration of the second step-up dose. (2.1)
•
For subcutaneous injection only. (2.2)
•
Administer pre-treatment medications as recommended. (2.3)
•
See Full Prescribing Information for instructions on preparation and administration. (2.6)
RECENT MAJOR CHANGES SECTION
RECENT MAJOR CHANGES
|
Dosage and Administration, Recommended Dosage (2.2) |
7/2025 |
|
Dosage and Administration, Restarting ELREXFIO After Dosage Delay (2.4) |
7/2025 |
|
Dosage and Administration, Preparation and Administration Instructions (2.6) |
7/2025 |

