Albuterol Sulate
These highlights do not include all the information needed to use Albuterol Sulfate HFA safely and effectively. See full prescribing information for Albuterol Sulfate HFA. Albuterol Sulfate HFA Inhalation Aerosol FOR ORAL INHALATION Initial U.S. Approval: 1981
825975b1-a4a9-78ca-e053-2a91aa0a3948
HUMAN PRESCRIPTION DRUG LABEL
Sep 2, 2025
NuCare Pharmaceuticals,Inc.
DUNS: 010632300
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
albuterol sulfate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (2)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL

SPL PATIENT PACKAGE INSERT SECTION
*Patient Information
Albuterol Sulfate HFA
Inhalation Aerosol
Read the Patient Information that comes with Albuterol Sulfate HFA Inhalation Aerosol before you start using it and each time you get a refill. There may be new information. This Patient Information does not take the place of talking to your healthcare provider about your medical condition or treatment.
What is Albuterol Sulfate HFA?
Albuterol Sulfate HFA is a prescription inhaled medicine used in people aged 4 years and older to:
- treat or prevent bronchospasm in people who have reversible obstructive airway disease
- prevent exercise-induced bronchospasm
It is not known if Albuterol Sulfate HFA is safe and effective in children younger than 4 years of age.
Who should not use Albuterol Sulfate HFA?
Do not use Albuterol Sulfate HFA if you are allergic to albuterol sulfate or any of the ingredients in Albuterol Sulfate HFA. See “What are the ingredients in Albuterol Sulfate HFA?” below for a complete list of ingredients.
What should I tell my healthcare provider before using Albuterol Sulfate HFA?
Tell your healthcare provider about all of your health conditions, including if you:
- have heart problems.
- have high blood pressure.
- have seizures.
- have thyroid problems.
- have diabetes.
- have low potassium levels in your blood.
- are allergic to any of the ingredients in Albuterol Sulfate HFA or any other medicines. See “What are the ingredients in Albuterol Sulfate HFA?” below for a complete list of ingredients.
- have any other medical conditions.
- are pregnant or planning to become pregnant. It is not known if Albuterol Sulfate HFA may harm your unborn baby.
- are breastfeeding. It is not known if the medicine in Albuterol Sulfate HFA passes into your milk and if it can harm your baby.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Albuterol Sulfate HFA and certain other medicines may interact with each other. This may cause serious side effects.
Especially tell your healthcare provider if you take:
- other inhaled medicines or asthma medicines
- beta-blocker medicines
- diuretics
- digoxin
- monoamine oxidase inhibitors
- tricyclic antidepressants
Ask your healthcare provider or pharmacist for a list of these medicines if you are not sure.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I use Albuterol Sulfate HFA?
Read the step-by-step instructions for using Albuterol Sulfate HFA at the end of this Patient Information.
*Do not use Albuterol Sulfate HFA unless your healthcare provider has taught you how to use the inhaler and you understand how to use it correctly.
- Children should use Albuterol Sulfate HFA with an adult’s help, as instructed by the child’s healthcare provider.
- Use Albuterol Sulfate HFA exactly as your healthcare provider tells you to use it.Do not use Albuterol Sulfate HFA more often than prescribed. *Do not increase your dose or take extra doses of Albuterol Sulfate HFA without first talking to your healthcare provider.
- Each dose of Albuterol Sulfate HFA should last up to 4 hours to 6 hours.
- Get medical help right away if Albuterol Sulfate HFA no longer helps your symptoms.
- Get medical help right away if your symptoms get worse or if you need to use your inhaler more often.
- While you are using Albuterol Sulfate HFA, use other inhaled medicines and asthma medicines only as directed by your healthcare provider.
- Call your healthcare provider if your asthma symptoms like wheezing and trouble breathing become worse over a few hours or days. Your healthcare provider may need to give you another medicine to treat your symptoms.
What are the possible side effects with Albuterol Sulfate HFA?
Albuterol Sulfate HFA can cause serious side effects, including:
*worsening trouble breathing, coughing, and wheezing (paradoxical bronchospasm). If this happens, stop using Albuterol Sulfate HFA and call your healthcare provider or get emergency help right away. Paradoxical bronchospasm is more likely to happen with your first use of a new canister of medicine. *heart problems, including faster heart rate and higher blood pressure *possible death in people with asthma who use too much Albuterol Sulfate HFA ***serious allergic reactions.**Call your healthcare provider or get emergency medical care if you get any of the following symptoms of a serious allergic reaction: * rash * hives * swelling of your face, mouth, and tongue * breathing problems *changes in laboratory blood levels (sugar, potassium)
Common side effects of Albuterol Sulfate HFA include:
- sore throat
- upper respiratory tract infection, including viral infection
- cough
- muscle pain
- your heart feels like it is pounding or racing (palpitations)
- chest pain
- fast heart rate
- shakiness
- nervousness
- dizziness
Tell your healthcare provider about any side effect that bothers you or that does not go away.
These are not all the side effects with Albuterol Sulfate HFA. Ask your healthcare provider or pharmacist for more information.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store Albuterol Sulfate HFA?
- Store Albuterol Sulfate HFA at room temperature between 68°F and 77°F (20°C and 25°C) with the mouthpiece down. ***The contents of your Albuterol Sulfate HFA are under pressure:**Do not puncture. Do not use or store near heat or open flame. Temperatures above 120°F may cause the canister to burst.
- Do not throw into fire or an incinerator.
- Store Albuterol Sulfate HFA in the unopened foil pouch and only open when ready for use. *Keep Albuterol Sulfate HFA and all medicines out of the reach of children.
General information about the safe and effective use of Albuterol Sulfate HFA
Medicines are sometimes prescribed for purposes not mentioned in a Patient Information leaflet. Do not use Albuterol Sulfate HFA for a condition for which it was not prescribed. Do not give your Albuterol Sulfate HFA to other people, even if they have the same condition that you have. It may harm them.
This Patient Information leaflet summarizes the most important information about Albuterol Sulfate HFA. If you would like more information, talk with your healthcare provider or pharmacist. You can ask your healthcare provider or pharmacist for information about Albuterol Sulfate HFA that was written for healthcare professionals.
For more information about Albuterol Sulfate HFA, call 1-888-825-5249.
What are the ingredients in Albuterol Sulfate HFA?
Active ingredient: albuterol sulfate
Inactive ingredient: propellant HFA-134a
Instructions for Use
For Oral Inhalation Only
Your Albuterol Sulfate HFA inhaler
- The metal canister holds the medicine.See Figure A.

- The canister has a counter to show how many sprays of medicine you have left. The number shows through a window in the back of the actuator.See Figure B.

- The counter starts at204. The number will count down by 1 each time you spray the inhaler. The counter will stop counting at000. *Do not try to change the numbers or take the counter off the metal canister. The counter cannot be reset, and it is permanently attached to the canister.
- The blue plastic actuator sprays the medicine from the canister. The actuator has a protective cap that covers the mouthpiece.See Figure A. Keep the protective cap on the mouthpiece when the canister is not in use. The strap keeps the cap attached to the actuator. *Do not use the actuator with a canister of medicine from any other inhaler. *Do not use an Albuterol Sulfate HFA canister with an actuator from any other inhaler.
Before using your Albuterol Sulfate HFA inhaler
- Take Albuterol Sulfate HFA out of the foil pouch just before you use it for the first time. Safely throw away the pouch and the drying packet that comes inside the pouch.
- The inhaler should be at room temperature before you use it.
- If your child needs to use Albuterol Sulfate HFA, watch your child closely to make sure your child uses the inhaler correctly. Your healthcare provider will show you how your child should use Albuterol Sulfate HFA.
Priming your Albuterol Sulfate HFA inhaler
*Before you use Albuterol Sulfate HFA for the first time, you must prime the inhaler so that you will get the right amount of medicine when you use it.
- To prime the inhaler, take the cap off the mouthpiece and shake the inhaler well. Then spray the inhaler 1 time into the air away from your face.See Figure C. Avoid spraying in eyes.

- Shake and spray the inhaler like this 3 more times to finish priming it. The counter should now read200.See Figure D.

- You must prime your inhaler again if you have not used it in more than 14 days or if you drop it. Take the cap off the mouthpiece and shake and spray the inhaler 4 times into the air away from your face.
How to use your Albuterol Sulfate HFA inhaler
Follow these steps every time you use Albuterol Sulfate HFA.
Step 1. Make sure the canister fits firmly in the actuator. The counter should show through the window in the actuator.
Shake the inhaler well before each spray.
Take the cap off the mouthpiece of the actuator. Look inside the mouthpiece for foreign objects, and take out any you see.
Step 2. Hold the inhaler with the mouthpiece down.See Figure E.

Step 3. Breathe out through your mouth and push as much air from your lungs as you can. Put the mouthpiece in your mouth and close your lips around it.** See Figure F.**

Step 4. Push the top of the canisterall the way down while you breathe in deeply and slowly through your mouth.See Figure F.
Step 5. After the spray comes out, take your finger off the canister. After you have breathed in all the way, take the inhaler out of your mouth and close your mouth.
Step 6. Hold your breath for about 10 seconds, or for as long as is comfortable.Breathe out slowly as long as you can.
If your healthcare provider has told you to use more sprays, wait 1 minute and shake the inhaler again. Repeat Steps 2 through Step 6.
Step 7. Put the cap back on the mouthpiece after every time you use the inhaler. Make sure it snaps firmly into place.
Cleaning your Albuterol Sulfate HFA inhaler
Clean your inhaler at least 1 time each week. You may not see any medicine build-up on the inhaler, but it is important to keep it clean so medicine build-up will not block the spray.** See Figure G.**

Step 8. Take the canister out of the actuator, and take the cap off the mouthpiece. The strap on the cap will stay attached to the actuator.
Step 9. Hold the actuator under the faucet and run warm water through it for about 30 seconds.** See Figure H.**

Step 10. Turn the actuator upside down and run warm water through the mouthpiece for about 30 seconds.** See Figure I.**

Step 11. Shake off as much water from the actuator as you can. Look into the mouthpiece to make sure any medicine build-up has been completely washed away. If there is any build-up, repeat Steps 9 and 10.
Step 12. Let the actuator air-dry overnight.See Figure J.

Step 13. When the actuator is dry, put the protective cap on the mouthpiece and then put the canister in the actuator and make sure it fits firmly. Shake the inhaler well, remove the cap, and spray the inhaler once into the air away from your face. (The counter will count down by 1 number.) Put the cap back on the mouthpiece.
If you need to use your inhaler before the actuator is completely dry:
- Shake as much water off the actuator as you can.
- Put the cap on the mouthpiece and then put the canister in the actuator and make sure it fits firmly.
- Shake the inhaler well and spray it 1 time into the air away from your face.
- Take your Albuterol Sulfate HFA dose as prescribed.
- Follow cleaning Steps 8 through 13 above.
Replacing your Albuterol Sulfate HFA inhaler:
*When the counter reads 020, you should refill your prescription or ask your healthcare provider if you need another prescription for Albuterol Sulfate HFA. *Throw the inhaler away when the counter reads000 or 12 months after you opened the foil pouch, whichever comes first. You should not keep using the inhaler when the counter reads000 because you will not receive the right amount of medicine. *Do not use the inhaler after the expiration date, which is on the packaging it comes in.
For correct use of your Albuterol Sulfate HFA inhaler, remember:
- The canister should always fit firmly in the actuator.
- Breathe in deeply and slowly to make sure you get all the medicine.
- Hold your breath for about 10 seconds after breathing in the medicine. Then breathe out fully.
- Always keep the protective cap on the mouthpiece when your inhaler is not in use.
- Always store your inhaler with the mouthpiece pointing down.
- Clean your inhaler at least 1 time each week.
If you have questions about Albuterol Sulfate HFA or how to use your inhaler, call 1-888-825-5249.
This Patient Information and Instructions for Use have been approved by the U.S. Food and Drug Administration.
Manufactured for:
Prasco Laboratories
Mason, OH 45040 USA
Manufactured by:
GlaxoSmithKline
Research Triangle Park, NC 27709
December 2015
VNT-PS:1PIL
DESCRIPTION SECTION
11 DESCRIPTION
The active component of Albuterol Sulfate HFA is albuterol sulfate, USP, the racemic form of albuterol and a relatively selective beta 2-adrenergic bronchodilator. Albuterol sulfate has the chemical name α 1-[( tert- butylamino)methyl]-4-hydroxy- m-xylene-α, α′-diol sulfate (2:1)(salt) and the following chemical structure:

Albuterol sulfate is a white crystalline powder with a molecular weight of 576.7, and the empirical formula is (C 13H 21NO 3) 2•H 2SO 4. It is soluble in water and slightly soluble in ethanol.
The World Health Organization recommended name for albuterol base is salbutamol.
Albuterol Sulfate HFA is a blue plastic inhaler with a blue strapcap containing a pressurized metered-dose aerosol canister fitted with a counter. Each canister contains a microcrystalline suspension of albuterol sulfate in propellant HFA-134a (1,1,1,2-tetrafluoroethane). It contains no other excipients.
After priming, each actuation of the inhaler delivers 120 mcg of albuterol sulfate, USP in 75 mg of suspension from the valve and 108 mcg of albuterol sulfate, USP from the mouthpiece (equivalent to 90 mcg of albuterol base from the mouthpiece).
Prime Albuterol Sulfate HFA before using for the first time, when the inhaler has not been used for more than 2 weeks, or when the inhaler has been dropped. To prime Albuterol Sulfate HFA, release 4 sprays into the air away from the face, shaking well before each spray.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Bronchospasm Associated with Asthma
Adult and Adolescent Subjects Aged 12 Years and Older: The efficacy of albuterol sulfate HFA was evaluated in two 12-week, randomized, double-blind, placebo controlled trials in subjects aged 12 years and older with mild to moderate asthma. These trials included a total of 610 subjects (323 males, 287 females). In each trial, subjects received 2 inhalations of albuterol sulfate HFA, CFC 11/12-propelled albuterol, or HFA-134a placebo 4 times daily for 12 weeks’ duration. Subjects taking the HFA-134a placebo inhaler also took albuterol sulfate HFA for asthma symptom relief on an as-needed basis. Some subjects who participated in these clinical trials were using concomitant inhaled steroid therapy. Efficacy was assessed by serial forced expiratory volume in 1 second (FEV 1). In each of these trials, 2 inhalations of albuterol sulfate HFA produced significantly greater improvement in FEV 1 over the pretreatment value than placebo. Results from the 2 clinical trials are described below.
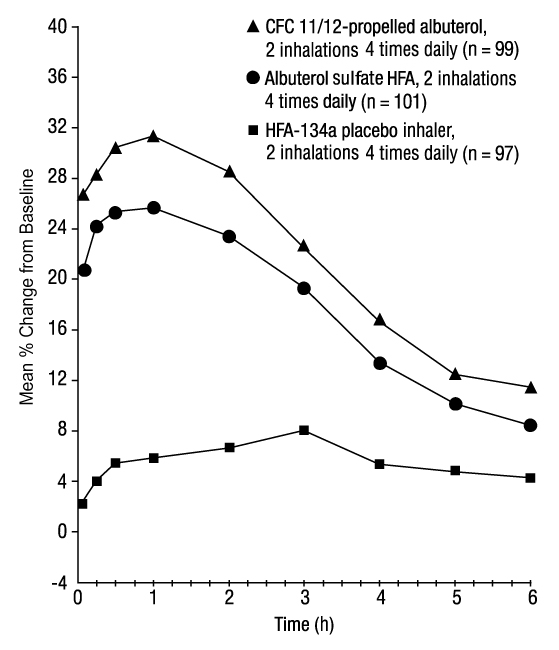
In a 12-week, randomized, double-blind trial, albuterol sulfate HFA (101 subjects) was compared with CFC 11/12-propelled albuterol (99 subjects) and an HFA-134a placebo inhaler (97 subjects) in adolescent and adult subjects aged 12 to 76 years with mild to moderate asthma. Serial FEV 1 measurements [shown below as percent change from test-day baseline at Day 1 (n = 297) and at Week 12 (n = 249)] demonstrated that 2 inhalations of albuterol sulfate HFA produced significantly greater improvement in FEV 1 over the pretreatment value than placebo.
FEV1 as Percent Change from Predose in a Large, 12-Week Clinical Trial
Day 1
Week 12
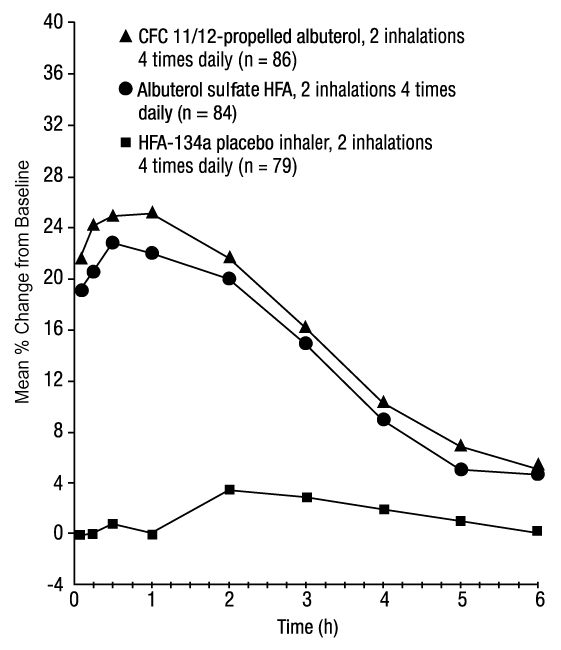
In the responder population (greater than or equal to 15% increase in FEV 1 within 30 minutes postdose) treated with albuterol sulfate HFA, the mean time to onset of a 15% increase in FEV 1 over the pretreatment value was 5.4 minutes, and the mean time to peak effect was 56 minutes. The mean duration of effect as measured by a 15% increase in FEV 1 over the pretreatment value was approximately 4 hours. In some subjects, duration of effect was as long as 6 hours.
The second 12-week randomized, double-blind trial was conducted to evaluate the efficacy and safety of switching subjects from CFC 11/12-propelled albuterol to albuterol sulfate HFA. During the 3-week run-in phase of the trial, all subjects received CFC 11/12-propelled albuterol. During the double- blind treatment phase, albuterol sulfate HFA (91 subjects) was compared to CFC 11/12-propelled albuterol (100 subjects) and an HFA-134a placebo inhaler (95 subjects) in adult and adolescent subjects with mild to moderate asthma. Serial FEV 1 measurements demonstrated that 2 inhalations of albuterol sulfate HFA produced significantly greater improvement in pulmonary function than placebo. The switching from CFC 11/12-propelled albuterol inhaler to albuterol sulfate HFA did not reveal any clinically significant changes in the efficacy profile.
In the 2 adult trials, the efficacy results from albuterol sulfate HFA were significantly greater than placebo and were clinically comparable to those achieved with CFC 11/12-propelled albuterol, although small numerical differences in mean FEV 1 response and other measures were observed. Physicians should recognize that individual responses to beta-adrenergic agonists administered via different propellants may vary and that equivalent responses in individual patients should not be assumed.
Pediatric Subjects Aged 4 to 11 Years: The efficacy of albuterol sulfate HFA was evaluated in one 2-week, randomized, double-blind, placebo-controlled trial in 135 pediatric subjects aged 4 to 11 years with mild to moderate asthma. In this trial, subjects received albuterol sulfate HFA, CFC 11/12-propelled albuterol, or HFA-134a placebo. Serial pulmonary function measurements demonstrated that 2 inhalations of albuterol sulfate HFA produced significantly greater improvement in pulmonary function than placebo and that there were no significant differences between the groups treated with albuterol sulfate HFA and CFC 11/12-propelled albuterol. In the responder population treated with albuterol sulfate HFA, the mean time to onset of a 15% increase in peak expiratory flow rate (PEFR) over the pretreatment value was 7.8 minutes, and the mean time to peak effect was approximately 90 minutes. The mean duration of effect as measured by a 15% increase in PEFR over the pretreatment value was greater than 3 hours. In some subjects, duration of effect was as long as 6 hours.
14.2 Exercise-Induced Bronchospasm
One controlled clinical trial in adult subjects with asthma (N = 24) demonstrated that 2 inhalations of albuterol sulfate HFA taken approximately 30 minutes prior to exercise significantly prevented exercise-induced bronchospasm (as measured by maximum percentage fall in FEV 1 following exercise) compared with an HFA-134a placebo inhaler. In addition, albuterol sulfate HFA was shown to be clinically comparable to a CFC 11/12-propelled albuterol inhaler for this indication.
