Luzu
These highlights do not include all the information needed to use LUZU safely and effectively. See full prescribing information for LUZU. LUZU (luliconazole) cream, for topical useInitial U.S. Approval: 2013
a7016010-ce43-4c09-8d21-aeb697ffed31
HUMAN PRESCRIPTION DRUG LABEL
Apr 1, 2020
Bausch Health US, LLC
DUNS: 831922468
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
LULICONAZOLE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 60 g Carton
NDC 99207-850-60
Rx only
LUZU**®**
(luliconazole) Cream, 1%
For Topical Use Only
Not for ophthalmic, oral, or intravaginal use
Keep Out of Reach of Children
Net. Wt. 60 g
OrthoDermatologics

ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In three Phase 3 clinical trials, 616 subjects were exposed to LUZU Cream, 1%: 305 with interdigital tinea pedis and 311 subjects with tinea cruris. Subjects with interdigital tinea pedis or tinea cruris applied LUZU Cream, 1% or vehicle cream once daily for 14 days or 7 days, respectively, to affected and adjacent areas. During clinical trials with LUZU Cream, 1%, the most common adverse reactions were application site reactions which occurred in less than 1% of subjects in both the LUZU and vehicle arms. Most adverse reactions were mild in severity.
A post-approval clinical trial was conducted in 75 subjects age 2 to <18 years old with tinea corporis. The adverse reactions in the LUZU Cream, 1% treated population were similar to the vehicle treated population.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postmarketing use of luliconazole cream, 1%: contact dermatitis and cellulitis. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The most common adverse reactions observed in clinical trials were application site reactions, which occurred in less than 1% of subjects. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Bausch Health US, LLC at 1-800-321-4576 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
LUZU Cream, 1% is an azole antifungal [see MICROBIOLOGY (12.4)].
12.2 Pharmacodynamics
At therapeutic doses, LUZU Cream, 1% is not expected to prolong QTc to any clinically relevant extent.
12.3 Pharmacokinetics
Luliconazole is the R enantiomer of a chiral molecule. The potential for interconversion between R and S enantiomers in humans has not been assessed. Information on the pharmacokinetics of luliconazole presented below refers to both R enantiomer and S enantiomer, if any, combined.
Luliconazole is >99% protein bound in plasma.
In a PK trial, 12 subjects with moderate to severe tinea pedis and 8 subjects with moderate to severe tinea cruris applied a mean daily amount of approximately 3.5 grams of LUZU Cream, 1% to the affected and surrounding areas once daily for 15 days. Plasma concentrations of luliconazole on Day 15 were measurable in all subjects and fluctuated little during the 24-hour interval. In subjects with tinea pedis, the mean ± SD of the maximum concentration (Cmax) was 0.40 ± 0.76 ng/mL after the first dose and 0.93 ± 1.23 ng/mL after the final dose. The mean time to reach Cmax (Tmax) was 16.9 ± 9.39 hours after the first dose and 5.8 ± 7.61 hours after the final dose. Exposure to luliconazole, as expressed by area under the concentration time curve (AUC0–24), was 6.88 ± 14.50 nghr/mL after the first dose and 18.74 ± 27.05 nghr/mL after the final dose. In subjects with tinea cruris, the mean ± SD Cmax was 4.91 ± 2.51 ng/mL after the first dose and 7.36 ± 2.66 ng/mL after the final dose. The mean Tmax was 21.0 ± 5.55 hours after the first dose and 6.5 ± 8.25 hours after the final dose. Exposure to luliconazole, as expressed by AUC0–24, was 85.1 ± 43.69 nghr/mL after the first dose and 121.74 ± 53.36 nghr/mL after the final dose.
PK data of luliconazole measured in adolescent subjects (12 to <18 years of age) with moderate to severe interdigital tinea pedis or moderate to severe tinea cruris as well as in subjects 2 to <18 years of age with tinea corporis are described in Specific Populations below.
Specific Populations
PK of luliconazole was assessed in 30 adolescent subjects 12 to <18 years of age with moderate to severe interdigital tinea pedis (N=15) or moderate to severe tinea cruris (N=15). Subjects with tinea pedis applied approximately 3 grams of LUZU Cream, 1% once daily to the affected area and adjacent skin for 15 days, while subjects with tinea cruris applied LUZU Cream, 1% once daily to the affected and adjacent skin for 8 days.
Generally, the systemic exposure of luliconazole was greater in the subjects with tinea cruris than tinea pedis. In subjects with tinea pedis, the systemic concentrations of luliconazole were quantifiable in all the subjects on Day 8 and Day 15. The mean ± SD Cmax was 1.80 ± 1.86 ng/mL after the first dose on Day 1, and 3.93 ± 1.67 ng/mL and 3.27 ± 1.71 ng/mL on Days 8 and 15, respectively. The mean ± SD AUC0-24 was 20.47 ± 14.47 nghr/mL after the first dose on Day 1, and 64.94 ± 32.47 nghr/mL and 60.38 ± 37.92 ng*hr/mL on Days 8 and Day 15, respectively. Like in adult subjects, the mean plasma concentrations of luliconazole in adolescent subjects on Days 8 and 15 were similar and fluctuated little during a 24-hour interval.
In subjects with moderate to severe tinea cruris, the systemic concentrations of luliconazole were quantifiable in all the subjects on Day 8. The mean ± SD Cmax was 9.80 ± 5.94 ng/mL after the first dose (Day 1) and 15.40 ± 13.62 ng/mL after the last dose (Day 8). The mean ± SD AUC0-24 was 157.07 ± 92.18 nghr/mL and 266.06 ± 236.07 nghr/mL, after the first dose (Day 1) and the last dose (Day 8).
In subjects with tinea corporis, the systemic concentrations of luliconazole were assessed at pre-dose and at 6 hours post-dose on the last day of treatment following once daily treatment with LUZU Cream, 1% for 7 days. The systemic concentrations were quantifiable in all the 12 subjects and the mean ± SD daily dose of LUZU Cream, 1% was 2.84 ± 1.82 g. The mean ± SD concentrations of luliconazole at 15 minutes prior to dosing and at 6 hours post-dose on Day 7 were 4.63 ± 2.93 ng/mL and 4.84 ± 3.33 ng/mL, respectively.
Drug Interactions
Results of in vitro studies indicated that therapeutic doses of LUZU Cream, 1% did not inhibit cytochrome P450 (CYP) enzymes 1A2, 2C9 and 2D6, but can inhibit the activity of CYP2B6, 2C8, 2C19, and 3A4. The most sensitive enzyme, CYP2C19, was further evaluated in an in vivo study using omeprazole as a probe substrate in adult subjects with moderate to severe interdigital tinea pedis and tinea cruris. The results showed that LUZU Cream, 1% applied at a daily amount of approximately 4 grams increased the omeprazole systemic exposure (AUC) by approximately 30% compared to the exposure of omeprazole administered alone. LUZU Cream, 1% is considered a weak inhibitor of CYP2C19. For tinea cruris, extrapolation from both in vitro inhibition studies and in vivo data in adults to the adolescent subjects showed that in some subjects, levels of luliconazole can approach or exceed those required to be a moderate inhibitor of CYP2C19.
Results of in vitro studies indicated that therapeutic doses of LUZU Cream, 1% did not induce CYP1A2, 2B6, and 3A4.
12.4 Microbiology
Mechanism of Action
Luliconazole is an antifungal that belongs to the azole class. Although the exact mechanism of action against dermatophytes is unknown, luliconazole appears to inhibit ergosterol synthesis by inhibiting the enzyme lanosterol demethylase. Inhibition of this enzyme’s activity by azoles results in decreased amounts of ergosterol, a constituent of fungal cell membranes, and a corresponding accumulation of lanosterol.
Mechanism of Resistance
To date, a mechanism of resistance to luliconazole has not been described.
LUZU Cream, 1% has been shown to be active against most isolates of the following fungi, both in vitro and in clinical infections as described in the INDICATIONS AND USAGE section:
Trichophyton rubrum
Epidermophyton floccosum
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
•
Inform patients that LUZU Cream, 1% is for topical use only. LUZU Cream, 1% is not intended for intravaginal or ophthalmic use.
Distributed by:
Bausch Health US, LLC
Bridgewater, NJ 08807 USA
Manufactured by:
Bausch Health Companies Inc.
Laval, Quebec H7L 4A8, Canada
U.S. Patent Numbers: 8,980,931; 9,012,484; 9,199,977 and 9,453,006
LUZU is a trademark of Bausch Health Companies Inc. or its affiliates.
© 2020 Bausch Health Companies Inc. or its affiliates
9438803
SPL PATIENT PACKAGE INSERT SECTION
PATIENT INFORMATION
LUZU**®**** (loo-zoo)**
(luliconazole) Cream, 1%
|
Important information: LUZU Cream is for use on skin only. Do not get LUZU Cream near or in your eyes, mouth or vagina. |
What is LUZU Cream?
LUZU Cream is a prescription medicine used on the skin (topical) to treat fungal infections in people with athlete’s foot that is between the toes, jock itch, and ringworm.
Before using LUZU Cream, tell your doctor about all of your medical conditions, including if you:
•
are pregnant or plan to become pregnant. It is not known if LUZU Cream will harm your unborn baby.
•
are breastfeeding or plan to breastfeed. It is not known if LUZU Cream passes into your breast milk.
Tell your doctor about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I use LUZU Cream?
•
Use LUZU Cream exactly as your doctor tells you to use it.
•
If you have athlete’s foot between the toes, apply a thin layer of LUZU Cream to the affected skin areas and to about 1 inch of the surrounding skin 1 time a day for 2 weeks.
•
If you have jock itch or ringworm, apply LUZU Cream to the affected skin areas and to about 1 inch of the surrounding skin 1 time a day for 1 week.
•
Wash your hands after you apply LUZU Cream.
What are the possible side effects of LUZU Cream?
LUZU Cream may cause skin reactions at the treatment site. Skin irritation may happen with LUZU Cream. Tell your doctor if you have any skin reactions on the areas of your skin treated with LUZU Cream.
These are not all the possible side effects of LUZU Cream.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store LUZU Cream?
•
Store LUZU Cream at room temperature between 68°F to 77°F (20°C to 25°C).
Keep LUZU Cream and all medicines out of the reach of children.
General information about the safe and effective use of LUZU Cream
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use LUZU Cream for a condition for which it was not prescribed. Do not give LUZU Cream to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or doctor for information about LUZU Cream that is written for health professionals.
What are the ingredients in LUZU Cream?
Active ingredient: luliconazole
Inactive ingredients: benzyl alcohol, butylated hydroxytoluene, cetostearyl alcohol, isopropyl myristate, medium-chain triglycerides, methylparaben, polysorbate 60, propylene glycol, purified water, sorbitan monostearate.
Distributed by:
Bausch Health US, LLC
Bridgewater, NJ 08807 USA
Manufactured by:
Bausch Health Companies Inc.
Laval, Quebec H7L 4A8, Canada
U.S. Patent Numbers: 8,980,931; 9,012,484; 9,199,977 and 9,453,006
LUZU is a trademark of Bausch Health Companies Inc. or its affiliates.
© 2020 Bausch Health Companies Inc. or its affiliates
For more information, call 1-800-321-4576.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: 04/2020
9438803
DESCRIPTION SECTION
11 DESCRIPTION
LUZU (luliconazole) Cream, 1% contains 1% luliconazole, an azole antifungal agent, in a white cream for topical application.
Luliconazole is (2E)-2-[(4R)-4-(2,4-dichlorophenyl)-1,3-dithiolan-2-ylidene]-2-imidazol-1-ylacetonitrile. Its structural formula is:
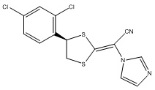
The molecular formula is C14H9Cl2N3S2 with a molecular weight of 354.28. Luliconazole is the R enantiomer and contains one chiral center. The double bond adjacent to the dithiolane group is in the E configuration.
LUZU Cream, 1% contains 10 mg of luliconazole per gram of cream in a vehicle consisting of benzyl alcohol, butylated hydroxytoluene, cetostearyl alcohol, isopropyl myristate, medium-chain triglycerides, methylparaben, polysorbate 60, propylene glycol, purified water, and sorbitan monostearate.
