Products (3)
Lasix
30698-066
NDA016273
NDA (C73594)
ORAL
February 9, 2023
Lasix
30698-060
NDA016273
NDA (C73594)
ORAL
February 9, 2023
Lasix
30698-067
NDA016273
NDA (C73594)
ORAL
February 9, 2023
Drug Labeling Information
DESCRIPTION SECTION
DESCRIPTION
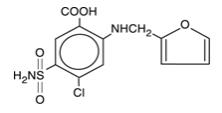
LASIX® is a diuretic which is an anthranilic acid derivative. LASIX tablets for oral administration contain furosemide as the active ingredient and the following inactive ingredients: lactose monohydrate NF, magnesium stearate NF, starch NF, talc USP, and colloidal silicon dioxide NF. Chemically, it is 4-chloro-N-furfuryl-5-sulfamoylanthranilic acid. LASIX is available as white tablets for oral administration in dosage strengths of 20, 40 and 80mg. Furosemide is a white to off-white odorless crystalline powder. It is practically insoluble in water, sparingly soluble in alcohol, freely soluble in dilute alkali solutions and insoluble in dilute acids.
The CAS Registry Number is 54-31-9.
The structural formula is as follows:
CLINICAL PHARMACOLOGY SECTION
CLINICAL PHARMACOLOGY
Investigations into the mode of action of LASIX have utilized micropuncture studies in rats, stop flow experiments in dogs and various clearance studies in both humans and experimental animals. It has been demonstrated that LASIX inhibits primarily the absorption of sodium and chloride not only in the proximal and distal tubu but also in the loop of Henle. The high degree of efficacy is largely due to the unique site of action. The action on the distal tubule is independent of any inhibitory effect on carbonic anhydrase and aldosterone.
Recent evidence suggests that furosemide glucuronide is the only or at least the major biotransformation product of furosemide in man. Furosemide is extensively bound to plasma proteins, mainly to albumin. Plasma concentrations ranging from 1 μg/mL to 400 μg/mL are 91% to 99% bound in healthy individuals. The unbound fraction averages 2.3% to 4.1% at therapeutic concentrations.
The onset of diuresis following oral administration is within 1 hour. The peak effect occurs within the first or second hour. The duration of diuretic effect is 6 to 8 hours.
In fasted normal men, the mean bioavailability of furosemide from LASIX Tablets and LASIX Oral Solution is 64% and 60%, respectively, of that from an intravenous injection of the drug. Although furosemide is more rapidly absorbed from the oral solution (50 minutes) than from the tablet (87 minutes), peak plasma levels and area under the plasma concentration-time curves do not differ significantly. Peak plasma concentrations increase with increasing dose but times-to-peak do not differ among doses. The terminal half-life of furosemide is approximately 2 hours.
Significantly more furosemide is excreted in urine following the IV injection than after the tablet or oral solution. There are no significant differences between the two oral formulations in the amount of unchanged drug excreted in urine.
Geriatric Population
Furosemide binding to albumin may be reduced in elderly patients. Furosemide is predominantly excreted unchanged in the urine. The renal clearance of furosemide after intravenous administration in older healthy male subjects (60 to 70 years of age) is statistically significantly smaller than in younger healthy male subjects (20 to 35 years of age). The initial diuretic effect of furosemide in older subjects is decreased relative to younger subjects (see PRECAUTIONS: Geriatric Use).
INDICATIONS & USAGE SECTION
INDICATIONS AND USAGE
Edema
LASIX is indicated in adults and pediatric patients for the treatment of edema associated with congestive heart failure, cirrhosis of the liver, and renal disease, including the nephrotic syndrome. LASIX is particularly useful when an agent with greater diuretic potential is desired.
Hypertension
Oral LASIX may be used in adults for the treatment of hypertension alone or in combination with other antihypertensive agents. Hypertensive patients who cannot be adequately controlled with thiazides will probably also not be adequately controlled with LASIX alone.
DOSAGE & ADMINISTRATION SECTION
DOSAGE AND ADMINISTRATION
Edema
Therapy should be individualized according to patient response to gain maximal therapeutic response and to determine the minimal dose needed to maintain that response.
Adults -- The usual initial dose of LASIX is 20 mg to 80mg given as a single dose. Ordinarily a prompt diuresis ensues. If needed, the same dose can be administered 6 to 8 hours later or the dose may be increased. The dose may be raised by 20 mg or 40mg and given not sooner than 6 to 8 hours after the previous dose until the desired diuretic effect has been obtained. The individually determined single dose should then be given once or twice daily (e.g., at 8 am and 2 pm). The dose of LASIX may be carefully titrated up to 600 mg/day in patients with clinically severe edematous states.
Edema may be most efficiently and safely mobilized by giving LASIX on 2 to 4 consecutive days each week.
When doses exceeding 80 mg/day are given for prolonged periods, careful clinical observation and laboratory monitoring are particularly advisable (see PRECAUTIONS: Laboratory Test).
Geriatric patients -- In general, dose selection for the elderly patient should be cautious, usually starting at the low end of the dosing range (see PRECAUTIONS: Geriatric Use).
Pediatric patients -- The usual initial dose of oral LASIX in pediatric patients is 2 mg/kg body weight, given as a single dose. If the diuretic response is not satisfactory after the initial dose, dosage may be increased by 1 or 2 mg/kg no sooner than 6 to 8 hours after the previous dose. Doses greater than 6 mg/kg body weight are not recommended. For maintenance therapy in pediatric patients, the dose should be adjusted to the minimum effective level.
Hypertension
Therapy should be individualized according to the patient’s response to gain maximal therapeutic response and to determine the minimal dose needed to maintain the therapeutic response.
Adults -- The usual initial dose of LASIX for hypertension is 80mg, usually divided into 40mg twice a day. Dosage should then be adjusted according to response. If response is not satisfactory, add other antihypertensive agents.
Changes in blood pressure must be carefully monitored when LASIX is used with other antihypertensive drugs, especially during initial therapy. To prevent excessive drop in blood pressure, the dosage of other agents should be reduced by at least 50% when LASIX is added to the regimen. As the blood pressure falls under the potentiating effect of LASIX, a further reduction in dosage or even discontinuation of other antihypertensive drugs may be necessary.
Geriatric patients -- In general, dose selection and dose adjustment for the elderly patient should be cautious, usually starting at the low end of the dosing range (see PRECAUTIONS: Geriatric Use).
ADVERSE REACTIONS SECTION
ADVERSE REACTIONS
Adverse reactions are categorized below by organ system and listed by decreasing severity.
|
Gastrointestinal System Reactions | |
|
1. hepatic encephalopathy in patients with hepatocellular insufficiency |
6. oral and gastric irritation |
|
2. pancreatitis |
8. diarrhea |
|
3. jaundice (intrahepatic cholestatic jaundice) |
9. constipation |
|
4. increased liver enzymes |
10. nausea |
|
5. anorexia |
11. vomiting |
|
Systemic Hypersensitivity Reactions | |
|
1. Severe anaphylactic or anaphylactoid reactions (e.g., with shock) |
3. interstitial nephritis |
|
2. systemic vasculitis | |
|
Central Nervous System Reactions | |
|
1. tinnitus and hearing loss |
5. headache |
|
2. paresthesias |
6. blurred vision |
|
3. vertigo |
7. xanthopsia |
|
4. dizziness | |
|
Hematologic Reactions | |
|
1. aplastic anemia |
5. leukopenia |
|
2. thrombocytopenia |
6. anemia |
|
3. agranulocytosis |
7. eosinophilia |
|
4. hemolytic anemia | |
|
Dermatologic-Hypersensitivity Reactions | |
|
1. toxic epidermal necrolysis |
7. bullous pemphigoid |
|
2. Stevens-Johnson Syndrome |
8. purpura |
|
4. drug rash with eosinophilia and systemic symptoms |
10. rash |
|
5. acute generalized exanthematous pustulosis |
12. urticaria |
|
Cardiovascular Reaction | |
|
1. Orthostatic hypotension may occur and be aggravated by alcohol, barbiturates, or narcotics. | |
|
2. Increase in cholesterol and triglyceride serum levels | |
|
Other Reactions | |
|
1. hyperglycemia |
6. restlessness |
|
2. glycosuria |
7. urinary bladder spasm |
|
3. hyperuricemia |
8. thrombophlebitis |
|
4. muscle spasm |
9. fever |
|
5. weakness |
Whenever adverse reactions are moderate or severe, LASIX dosage should be reduced or therapy withdrawn.
To report SUSPECTED ADVERSE REACTIONS, contact Validus Pharmaceuticals LLC at
1-866-982-5438 (1-866-9VALIDUS) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
