Phenazopyridine Hydrochloride
PHENAZOPYRIDINE HYDROCHLORIDE TABLETS, USP Rev 12/21 Rx Only CAUTION: Federal law prohibits dispensing without prescription
7e131f69-d259-404d-a859-0497f5cbe856
HUMAN PRESCRIPTION DRUG LABEL
Jan 19, 2024
REMEDYREPACK INC.
DUNS: 829572556
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Phenazopyridine Hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
DRUG: Phenazopyridine Hydrochloride
GENERIC: Phenazopyridine Hydrochloride
DOSAGE: TABLET
ADMINSTRATION: ORAL
NDC: 70518-0218-0
NDC: 70518-0218-1
NDC: 70518-0218-2
COLOR: brown
SHAPE: ROUND
SCORE: No score
SIZE: 7 mm
IMPRINT: 114
PACKAGING: 6 in 1 BOTTLE PLASTIC
PACKAGING: 30 in 1 BLISTER PACK
PACKAGING: 10 in 1 BOTTLE PLASTIC
ACTIVE INGREDIENT(S):
- PHENAZOPYRIDINE HYDROCHLORIDE 100mg in 1
INACTIVE INGREDIENT(S):
- HYPROMELLOSE 2910 (15 MPA.S)
- POLYETHYLENE GLYCOL 400
- CROSCARMELLOSE SODIUM
- MAGNESIUM STEARATE
- MICROCRYSTALLINE CELLULOSE
- STARCH, CORN
- SILICON DIOXIDE
- POVIDONE K30


DESCRIPTION SECTION
DESCRIPTION
Phenazopyridine Hydrochloride is dark brown fine granular powder. It has a specific local analgesic effect in the urinary tract, promptly relieving burning and pain.
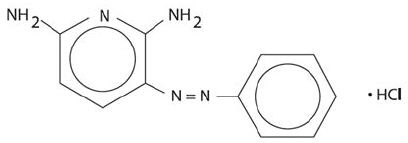
Phenazopyridine HCl tablets contain the following inactive ingredients: hypromellose, polyethylene glycol, croscarmellose sodium, magnesium stearate, microcrystalline cellulose, pregelatinized starch, maize(corn) starch, silicone dioxide, polyvinylpyrrolidone.
DOSAGE & ADMINISTRATION SECTION
DOSAGE AND ADMINISTRATION
100 mg Tablets: Average adult dosage is two tablets 3 times a day after meals.
When used concomitantly with an antibacterial agent for the treatment of a urinary tract infection, the administration of Phenazopyridine HCl should not exceed 2 days.
To report SUSPECTED ADVERSE REACTIONS, contact Winder Laboratories, LLC at 1-770-307-0703, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
