Mekinist
These highlights do not include all the information needed to use MEKINIST safely and effectively. See full prescribing information for MEKINIST. MEKINIST (trametinib) tablets, for oral useMEKINIST (trametinib) for oral solutionInitial U.S. Approval: 2013
0002ad27-779d-42ab-83b5-bc65453412a1
HUMAN PRESCRIPTION DRUG LABEL
Mar 29, 2024
Novartis Pharmaceuticals Corporation
DUNS: 002147023
Products 5
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
trametinib
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
trametinib
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
trametinib
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
trametinib
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
trametinib
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NDC 0078-1161-47
Rx only
Mekinist®
(trametinib)
for Oral Solution
0.05 mg per mL*
90 mL (when reconstituted)
To Pharmacist: Reconstitute before
dispensing*. Dispense with the enclosed
Patient Information and Instructions
for Use.
For administration by caregivers only.
NOVARTIS

CONTRAINDICATIONS SECTION
4** CONTRAINDICATIONS**
None.
None. (4)
DRUG INTERACTIONS SECTION
7** DRUG INTERACTIONS**
MEKINIST is indicated for use in combination with dabrafenib. Refer to the dabrafenib prescribing information for additional risk information that applies to combination use treatment.
INFORMATION FOR PATIENTS SECTION
17** PATIENT COUNSELING INFORMATION**
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
New Primary Cutaneous and Non-cutaneous Malignancies
Advise patients that MEKINIST administered with dabrafenib can result in the development of new primary cutaneous and non-cutaneous malignancies. Advise patients to contact their healthcare provider immediately for any new lesions, changes to existing lesions on their skin, or other signs and symptoms of malignancies [see Warnings and Precautions (5.1)].
Hemorrhage
Advise patients that MEKINIST administered with dabrafenib increases the risk of intracranial and gastrointestinal hemorrhage. Advise patients to contact their healthcare provider to seek immediate medical attention for signs or symptoms of unusual bleeding or hemorrhage [see Warnings and Precautions (5.2)].
Colitis and Gastrointestinal Perforation
Advise patients that MEKINIST can cause colitis and gastrointestinal perforation and to contact their healthcare provider for signs or symptoms of colitis or gastrointestinal perforation [see Warnings and Precautions (5.3)].
Venous Thromboembolic Events
Advise patients that MEKINIST administered with dabrafenib increases the risks of PE and DVT. Advise patients to seek immediate medical attention for sudden onset of difficulty breathing, leg pain, or swelling [see Warnings and Precautions (5.4)].
Cardiomyopathy
Advise patients that MEKINIST can cause cardiomyopathy and to immediately report any signs or symptoms of heart failure to their healthcare provider [see Warnings and Precautions (5.5)].
Ocular Toxicities
Advise patients that MEKINIST can cause severe visual disturbances that can lead to blindness and to contact their healthcare provider if they experience any changes in their vision [see Warnings and Precautions (5.6)].
Interstitial Lung Disease/Pneumonitis
Advise patients that MEKINIST can cause ILD (or pneumonitis). Advise patients to contact their healthcare provider as soon as possible if they experience signs, such as cough or dyspnea [see Warnings and Precautions (5.7)].
Serious Febrile Reactions
Advise patients that MEKINIST administered with dabrafenib can cause serious febrile reactions. Instruct patients to contact their healthcare provider if they develop a fever while taking MEKINIST with dabrafenib [see Warnings and Precautions (5.8)].
Serious Skin Toxicities
Advise patients that MEKINIST can cause serious skin toxicities, which may require hospitalization, and to contact their healthcare provider for progressive or intolerable rash. Advise patients to contact their healthcare provider immediately if they develop signs and symptoms of a severe skin reaction [see Warnings and Precautions (5.9)].
Hypertension
Advise patients that MEKINIST can cause hypertension and that they need to undergo blood pressure monitoring and to contact their healthcare provider if they develop symptoms of hypertension, such as severe headache, blurry vision, or dizziness [see Adverse Reactions (6.1)].
Diarrhea
Advise patients that MEKINIST often causes diarrhea which may be severe in some cases. Inform patients of the need to contact their healthcare provider if severe diarrhea occurs during treatment [see Adverse Reactions (6.1)].
Embryo-Fetal Toxicity
- Advise pregnant women and males of reproductive potential of the potential risk to a fetus [see Warnings and Precautions (5.13), Use in Specific Populations (8.1, 8.3)].
- Advise females to contact their healthcare provider of a known or suspected pregnancy.
- Advise females of reproductive potential to use effective contraception during treatment with MEKINIST and for 4 months after the last dose.
- Advise male patients with female partners of reproductive potential to use condoms during treatment with MEKINIST and for 4 months after the last dose.
Lactation
Advise women not to breastfeed during treatment with MEKINIST and for 4 months after the last dose [see Use in Specific Populations (8.2)].
Infertility
Advise females of reproductive potential of the potential risk for impaired fertility [see Use in Specific Populations (8.3)].
Administration
Instruct patients to take MEKINIST at least 1 hour before or at least 2 hours after a meal [see Dosage and Administration (2.3)].
THxID® BRAF assay is a trademark of bioMérieux.
Oncomine™ Dx Target Test is a trademark of Life Technologies Corporation, a part of Thermo Fisher Scientific Inc.
Distributed by:
Novartis Pharmaceuticals Corporation
East Hanover, New Jersey 07936
© Novartis
T2024-25
RECENT MAJOR CHANGES SECTION
RECENT MAJOR CHANGES
|
Indications and Usage, BRAF V600E Mutation-Positive Unresectable or Metastatic Solid Tumors (1.5) |
8/2023 |
|
Warnings and Precautions, Hemophagocytic Lymphohistiocytosis (5.12) |
5/2023 |
DOSAGE & ADMINISTRATION SECTION
2** DOSAGE AND ADMINISTRATION**
2.1 Patient Selection
Melanoma
- Confirm the presence of BRAF V600E or V600K mutation in tumor specimens prior to initiation of treatment with MEKINIST as a single agent or in combination with dabrafenib [see Clinical Studies (14.1, 14.2)].
- Information on FDA-approved tests for the detection of BRAF V600 mutations in melanoma is available at: http://www.fda.gov/CompanionDiagnostics.
NSCLC
- Confirm the presence of BRAF V600E mutation in tumor specimens prior to initiation of treatment with MEKINIST and dabrafenib [see Clinical Studies (14.3)].
- Information on FDA-approved tests for the detection of BRAF V600E mutations in NSCLC is available at: http://www.fda.gov/CompanionDiagnostics.
ATC
- Confirm the presence of BRAF V600E mutation in tumor specimens prior to initiation of treatment with MEKINIST and dabrafenib [see Clinical Studies (14.4)]. An FDA-approved test for the detection of BRAF V600E mutation in ATC is not currently available.
Solid Tumors
- Confirm the presence of BRAF V600E mutation in tumor specimens prior to initiation of treatment with MEKINIST and dabrafenib [see Clinical Studies (14.6)]. An FDA-approved test for the detection of BRAF V600E mutation in solid tumors other than melanoma and NSCLC is not currently available.
Low-Grade Glioma
- Confirm the presence of BRAF V600E mutation in tumor specimens prior to initiation of treatment with MEKINIST and dabrafenib [see Clinical Studies (14.7)]. An FDA-approved test for the detection of BRAF V600E mutation in LGG is not currently available.
2.2 Recommended Dosage
MEKINIST Tablets
Adult Patients
The recommended dosage for MEKINIST tablets in adult patients is 2 mg orally taken once daily [see Dosage and Administration (2.3)].
Pediatric Patients
The recommended dosage for MEKINIST tablets in pediatric patients who weigh at least 26 kg is based on body weight (Table 1) [see Dosage and Administration (2.3)]. A recommended dosage of MEKINIST tablets has not been established in patients who weigh less than 26 kg.
Table 1. Recommended Dosage for MEKINIST Tablets in Pediatric Patients (Weight-based)|
Body Weight |
Recommended Dosage |
|
26 to 37 kg |
1 mg orally once daily |
|
38 to 50 kg |
1.5 mg orally once daily |
|
51 kg or greater |
2 mg orally once daily |
MEKINIST for Oral Solution
The recommended dosage for MEKINIST for oral solution is based on body weight (Table 2) [see Dosage and Administration (2.3)].
Table 2. Recommended Dosage for MEKINIST for Oral Solution (Weight- based)|
Body Weight |
Recommended Dosage |
|
8 kg |
0.3 mg (6 mL) |
|
9 kg |
0.35 mg (7 mL) |
|
10 kg |
0.35 mg (7 mL) |
|
11 kg |
0.4 mg (8 mL) |
|
12 to 13 kg |
0.45 mg (9 mL) |
|
14 to 17 kg |
0.55 mg (11 mL) |
|
18 to 21 kg |
0.7 mg (14 mL) |
|
22 to 25 kg |
0.85 mg (17 mL) |
|
26 to 29 kg |
0.9 mg (18 mL) |
|
30 to 33 kg |
1 mg (20 mL) |
|
34 to 37 kg |
1.15 mg (23 mL) |
|
38 to 41 kg |
1.25 mg (25 mL) |
|
42 to 45 kg |
1.4 mg (28 mL) |
|
46 to 50 kg |
1.6 mg (32 mL) |
|
≥ 51 kg |
2 mg (40 mL) |
- The recommended duration of treatment for patients with unresectable or metastatic melanoma or solid tumors, metastatic NSCLC, or locally advanced or metastatic anaplastic thyroid cancer is until disease progression or unacceptable toxicity.
- The recommended duration of treatment in the adjuvant melanoma setting is until disease recurrence or unacceptable toxicity for up to 1 year.
- The recommended duration of treatment for pediatric patients with LGG is until disease progression or until unacceptable toxicity.
Refer to the dabrafenib prescribing information for recommended dabrafenib dosing information.
2.3 Administration
- Take MEKINIST at the same time each day, approximately 24 hours apart.
- Take MEKINIST at least 1 hour before or 2 hours after a meal [see Clinical Pharmacology (12.3)].
- Do not take a missed dose of MEKINIST within 12 hours of the next dose of MEKINIST.
- If vomiting occurs after MEKINIST administration, do not take an additional dose. Take the next dose at its scheduled time.
MEKINIST Tablets
- Do not crush or break MEKINIST tablets.
MEKINIST for Oral Solution
- MEKINIST for oral solution is intended for administration by a caregiver. Prior to use of the oral solution, ensure caregivers receive training on proper dosing and administration of MEKINIST for oral solution.
Preparation and Administration
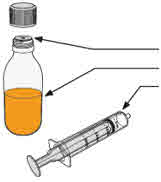
- To prepare MEKINIST for oral solution, tap the bottle until powder flows freely. Add 90 mL distilled or purified water to the powder in the bottle and invert or gently shake the bottle with re-attached cap for up to 5 minutes until powder is fully dissolved yielding a clear solution. Separate the bottle adapter from the oral syringe. Insert bottle adapter into bottle neck after reconstitution of the solution. Write the discard after date. Once reconstituted, MEKINIST for oral solution can be used for 35 days.
- The final concentration of the solution is 0.05 mg/mL.
- Administer MEKINIST for oral solution from an oral syringe or feeding tube (4 French gauge or larger).
- After reconstitution, store in original bottle below 25°C (77°F) and do not freeze.
2.4 Dosage Modifications for Adverse Reactions
Dose reductions for adverse reactions associated with MEKINIST are presented in Tables 3 and 4.
Table 3. Recommended Dosage Reductions for MEKINIST Tablets for Adverse Reactions|
Recommended Dosage |
1 mg orally once daily |
1.5 mg orally once daily |
2 mg orally once daily |
|
First dose reduction |
0.5 mg orally once daily |
1 mg orally once daily |
1.5 mg orally once daily |
|
Second dose reduction |
N/A |
0.5 mg orally once daily |
1 mg orally once daily |
|
Subsequent modification |
Permanently discontinue MEKINIST tablets if unable to tolerate a maximum of two dose reductions. |
|
Body Weight |
First Dose Reduction |
Second Dose Reduction |
|
8 kg |
0.25 mg (5 mL) |
0.15 mg (3 mL) |
|
9 kg |
0.25 mg (5 mL) |
0.2 mg (4 mL) |
|
10 kg |
0.25 mg (5 mL) |
0.2 mg (4 mL) |
|
11 kg |
0.3 mg (6 mL) |
0.2 mg (4 mL) |
|
12 to 13 kg |
0.35 mg (7 mL) |
0.25 mg (5 mL) |
|
14 to 17 kg |
0.4 mg (8 mL) |
0.3 mg (6 mL) |
|
18 to 21 kg |
0.55 mg (11 mL) |
0.35 mg (7 mL) |
|
22 to 25 kg |
0.65 mg (13 mL) |
0.45 mg (9 mL) |
|
26 to 29 kg |
0.7 mg (14 mL) |
0.45 mg (9 mL) |
|
30 to 33 kg |
0.75 mg (15 mL) |
0.5 mg (10 mL) |
|
34 to 37 kg |
0.85 mg (17 mL) |
0.6 mg (12 mL) |
|
38 to 41 kg |
0.95 mg (19 mL) |
0.65 mg (13 mL) |
|
42 to 45 kg |
1.05 mg (21 mL) |
0.7 mg (14 mL) |
|
46 to 50 kg |
1.2 mg (24 mL) |
0.8 mg (16 mL) |
|
≥ 51 kg |
1.5 mg (30 mL) |
1 mg (20 mL) |
|
Permanently discontinue MEKINIST for oral solution if unable to tolerate a maximum of two dose reductions. |
Dosage modifications for adverse reactions associated with MEKINIST are presented in Table 5.
Table 5. Recommended Dosage Modifications for MEKINIST for Adverse Reactions|
aNational Cancer Institute Common Terminology Criteria for Adverse Events (NCI
CTCAE) version 4.0. | |
|
Severity of Adverse Reaction****a |
Dosage Modification for MEKINIST****b |
|
Hemorrhage [see Warnings and Precautions (5.2)] | |
|
Withhold MEKINIST.
|
|
Permanently discontinue MEKINIST. |
|
Venous Thromboembolic Events [see Warnings and Precautions (5.4)] | |
|
Withhold MEKINIST for up to 3 weeks.
|
|
Permanently discontinue MEKINIST. |
|
Cardiomyopathy [see Warnings and Precautions (5.5)] | |
|
Withhold MEKINIST for up to 4 weeks.
|
|
Permanently discontinue MEKINIST. |
|
Ocular Toxicities [see Warnings and Precautions (5.6)] | |
|
Withhold MEKINIST for up to 3 weeks.
|
|
Permanently discontinue MEKINIST. |
|
Pulmonary [see Warnings and Precautions (5.7)] | |
|
Permanently discontinue MEKINIST. |
|
Febrile Reactions [see Warnings and Precautions (5.8)] | |
|
Withhold MEKINIST until fever resolves, then resume MEKINIST at same or lower dose. |
|
Or
|
|
Skin Toxicities [see Warnings and Precautions (5.9)] | |
|
Withhold MEKINIST for up to 3 weeks.
|
|
Permanently discontinue MEKINIST. |
|
Other Adverse Reactionsc | |
|
Withhold MEKINIST.
|
|
Or
|
|
Permanently discontinue MEKINIST. |
Refer to the dabrafenib prescribing information for dose modifications for adverse reactions associated with dabrafenib.
- The recommended dosage of MEKINIST in adult patients is 2 mg orally once daily. The recommended dosage for MEKINIST in pediatric patients is based on body weight. Take MEKINIST at least 1 hour before or at least 2 hours after a meal. (2)
DOSAGE FORMS & STRENGTHS SECTION
3** DOSAGE FORMS AND STRENGTHS**
MEKINIST tablets:
- 0.5 mg tablets: Yellow, modified oval, biconvex, film-coated tablets with ‘GS’ debossed on one face and ‘TFC’ on the opposing face.
- 0.5 mg tablets: Yellow, ovaloid, biconvex, unscored film-coated tablets with beveled edges and with the Novartis logo debossed on one side and ‘TT’ on the other side.
- 2 mg tablets: Pink, round, biconvex, film-coated tablets with ‘GS’ debossed on one face and ‘HMJ’ on the opposing face.
- 2 mg tablets: Pink, round, biconvex, unscored film-coated tablets with beveled edges and with the Novartis logo debossed on one side and ‘LL’ on the other side.
MEKINIST for oral solution:
- White to almost white powder containing 4.7 mg of trametinib per bottle. Each mL of reconstituted strawberry-flavored trametinib solution contains 0.05 mg of trametinib.
MEKINIST Tablets: 0.5 mg, 2 mg (3)
MEKINIST for Oral Solution: 4.7 mg (3)
USE IN SPECIFIC POPULATIONS SECTION
8** USE IN SPECIFIC POPULATIONS**
8.1 Pregnancy
Risk Summary
Based on its mechanism of action [see Clinical Pharmacology (12.1)] and findings from animal reproduction studies, MEKINIST can cause fetal harm when administered to a pregnant woman. There is insufficient data in pregnant women exposed to MEKINIST to assess the risks. Trametinib was embryotoxic and abortifacient in rabbits at doses greater than or equal to those resulting in exposures approximately 0.3 times the human exposure at the recommended adult clinical dose (see Data). Advise pregnant women of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
In reproductive toxicity studies, administration of trametinib to rats during the period of organogenesis resulted in decreased fetal weights at doses greater than or equal to 0.031 mg/kg/day [approximately 0.3 times the human exposure at the recommended adult dose based on area under the curve (AUC)]. In rats, at a dose resulting in exposures 1.8-fold higher than the human exposure at the recommended adult dose, there was maternal toxicity and an increase in post-implantation loss.
In pregnant rabbits, administration of trametinib during the period of organogenesis resulted in decreased fetal body weight and increased incidence of variations in ossification at doses greater than or equal to 0.039 mg/kg/day (approximately 0.08 times the human exposure at the recommended adult dose based on AUC). In rabbits administered trametinib at 0.15 mg/kg/day (approximately 0.3 times the human exposure at the recommended adult dose based on AUC) there was an increase in post-implantation loss, including total loss of pregnancy, compared with control animals.
8.2 Lactation
Risk Summary
There are no data on the presence of trametinib in human milk, or the effects of trametinib on the breastfed child or on milk production. Because of the potential for serious adverse reactions in breastfed children, advise women not to breastfeed during treatment with MEKINIST and for 4 months following the last dose.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Verify pregnancy status in females of reproductive potential prior to initiating MEKINIST.
Contraception
Based on data from animal studies and its mechanism of action, MEKINIST can cause fetal harm when administered to pregnant women [see Use in Specific Populations (8.1)].
Females
Advise female patients of reproductive potential to use effective contraception during treatment with MEKINIST and for 4 months after the last dose.
Males
To avoid potential drug exposure to pregnant partners and female partners of reproductive potential, advise male patients (including those who have had vasectomies) with female partners of reproductive potential to use condoms during treatment with MEKINIST and for 4 months after the last dose.
Infertility
Females
Advise female patients of reproductive potential that MEKINIST may impair fertility. Increased follicular cysts and decreased corpora lutea were observed in female rats at dose exposures equivalent to 0.3 times the human exposure at the recommended adult dose [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
BRAF V600E Mutation-Positive Unresectable or Metastatic Solid Tumors and LGG
The safety and effectiveness of MEKINIST in combination with dabrafenib have been established in pediatric patients 1 year of age and older with unresectable or metastatic solid tumors with BRAF V600E mutation who have progressed following prior treatment and have no satisfactory alternative treatment options; or with LGG with BRAF V600E mutation who require systemic therapy. Use of MEKINIST in combination with dabrafenib for these indications is supported by evidence from studies X2101 and G2201 that enrolled 171 patients (1 to < 18 years) with BRAF V600 mutation-positive advanced solid tumors, of which 4 (2.3%) patients were 1 to < 2 years of age, 39 (23%) patients were 2 to < 6 years of age, 54 (32%) patients were 6 to < 12 years of age, and 74 (43%) patients were 12 to < 18 years of age [see Adverse Reactions (6.1), Clinical Pharmacology (12.3), Clinical Studies (14.6, 14.7)].
The safety and effectiveness of MEKINIST in combination with dabrafenib have not been established for these indications in pediatric patients less than 1 year old.
The safety and effectiveness of MEKINIST as a single agent in pediatric patients have not been established.
Juvenile Animal Toxicity Data
In a repeat-dose toxicity study in juvenile rats, decreased bone length and corneal dystrophy were observed at doses resulting in exposures as low as 0.3 times the human exposure at the recommended adult dose based on AUC. Additionally, a delay in sexual maturation was noted at doses resulting in exposures as low as 1.6 times the human exposure at the recommended adult dose based on AUC.
8.5 Geriatric Use
Of the 214 patients with melanoma who received single agent MEKINIST in the METRIC study, 27% were aged 65 years and older and 4% were over 75 years old [see Clinical Studies (14.1)]. This study of single agent MEKINIST in melanoma did not include sufficient numbers of geriatric patients to determine whether they respond differently from younger adults.
Of the 994 patients with melanoma who received MEKINIST plus dabrafenib in the COMBI-d, COMBI-v, and COMBI-AD studies [see Clinical Studies (14.1, 14.2)], 21% were aged 65 years and older and 5% were aged 75 years and older. No overall differences in the effectiveness of MEKINIST plus dabrafenib were observed in geriatric patients as compared to younger adults across these melanoma studies. The incidences of peripheral edema (26% vs. 12%) and anorexia (21% vs. 9%) increased in geriatric patients as compared to younger adults in these studies.
Of the 93 patients with NSCLC who received MEKINIST in Study BRF113928, there were insufficient numbers of geriatric patients aged 65 and older to determine whether they respond differently from younger adults [see Clinical Studies (14.4)].
Of the 26 patients with ATC who received MEKINIST in Study BRF117019, 77% were aged 65 years and older and 31% were aged 75 years and older [see Clinical Studies (14.4)]. This study in ATC did not include sufficient numbers of younger adults to determine whether they respond differently compared to geriatric patients.
8.6 Hepatic Impairment
No dose adjustment is recommended in patients with mild (bilirubin ≤ upper limit of normal (ULN) and aspartate aminotransferase (AST) > ULN or bilirubin
1x to 1.5x ULN and any AST) hepatic impairment.
A recommended dosage of MEKINIST has not been established for patients with moderate (bilirubin > 1.5x to 3x ULN and any AST) or severe (bilirubin > 3x to 10x ULN and any AST) hepatic impairment. Consider the risk-benefit profile of MEKINIST related to dosing prior to determining whether to administer MEKINIST to patients with moderate or severe hepatic impairment.
In patients with moderate hepatic impairment, 3 patients who received a starting dose of 1.5 mg orally once daily and two patients who received a starting dose of 2 mg orally once daily did not experience dose limiting toxicities (DLTs) during the first cycle of therapy.
In patients with severe hepatic impairment, 3 patients who received a starting dose of 1 mg orally once daily did not experience DLTs during the first cycle; one patient who received a starting dose of 1.5 mg orally once daily experienced a DLT (grade 3 acneiform rash).
Compared to patients with normal hepatic function, there was no increase in exposure of trametinib in patients with moderate or severe hepatic impairment [see Clinical Pharmacology (12.3)].
- Lactation: Do not breastfeed. (8.2)
- Females and Males of Reproductive Potential: May impair fertility. Counsel patients on pregnancy planning and prevention. (8.3)
OVERDOSAGE SECTION
10** OVERDOSAGE**
The highest doses of MEKINIST evaluated in clinical trials were 4 mg orally once daily and 10 mg administered orally once daily on 2 consecutive days followed by 3 mg once daily. In seven patients treated on one of these two schedules, there were two cases of RPEDs for an incidence of 28%.
Since trametinib is highly bound to plasma proteins, hemodialysis is likely to be ineffective in the treatment of overdose with MEKINIST.
HOW SUPPLIED SECTION
16** HOW SUPPLIED/STORAGE AND HANDLING**
MEKINIST Tablets:
0.5 mg tablets: Yellow, modified oval, biconvex, film-coated tablets with ‘GS’ debossed on one face and ‘TFC’ on the opposing face and are available in bottles of 30 (NDC 0078-0666-15).
0.5 mg tablets: Yellow, ovaloid, biconvex, unscored film-coated tablets with beveled edges and with the Novartis logo debossed on one side and ‘TT’ on the other side; available in bottles of 30 (NDC 0078-1105-15).
2 mg tablets: Pink, round, biconvex, film-coated tablets with ‘GS’ debossed on one face and ‘HMJ’ on the opposing face and are available in bottles of 30 (NDC 0078-0668-15).
2 mg tablets: Pink, round, biconvex, unscored film-coated tablets with beveled edges and with the Novartis logo debossed on one side and ‘LL’ on the other side; available in bottles of 30 (NDC 0078-1112-15).
Store refrigerated at 2°C to 8°C (36°F to 46°F). Dispense in original bottle. Do not remove desiccant. Protect from moisture and light. Do not place medication in pill boxes.
MEKINIST for Oral Solution:
White or almost white powder in amber glass bottles, co-packaged with a press- in bottle adapter and an oral syringe. Each bottle contains 4.7 mg of trametinib equivalent to 5.3 mg trametinib dimethyl sulfoxide. Each mL of reconstituted strawberry flavored trametinib solution contains 0.05 mg of trametinib non-solvated parent. (NDC 0078-1161-47).
Store refrigerated at 2°C to 8°C (36°F to 46°F). Store in the original carton to protect from light and moisture.
After reconstitution, store in the original bottle below 25°C (77°F) and do not freeze.
Discard any unused solution 35 days after reconstitution.
SPL PATIENT PACKAGE INSERT SECTION
|
This Patient Information has been approved by the U.S. Food and Drug Administration. |
Revised: March 2024 |
|
Patient Information | |
|
MEKINIST**®**** (MEK-in-ist)** |
MEKINIST**®**** (MEK-in-ist)** |
|
Important information: If your healthcare provider prescribes MEKINIST to be taken or given with dabrafenib, also read the Medication Guide that comes with dabrafenib. | |
|
What is the most important information I should know about MEKINIST?
Your healthcare provider should check your skin before treatment with MEKINIST
and dabrafenib, every 2 months during treatment with MEKINIST and dabrafenib,
and for up to 6 months after you stop taking MEKINIST and dabrafenib to look
for any new skin cancers. | |
|
What is MEKINIST?
MEKINIST should not be used to treat people who already have received a BRAF inhibitor for treatment of their melanoma, and it did not work or is no longer working.
MEKINIST is not for use in treating people with colorectal cancer. | |
|
Before you take or give MEKINIST, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including
prescription and over-the-counter medicines, vitamins, and herbal supplements. | |
|
How should I take or give MEKINIST?
MEKINIST tablets:
MEKINIST for oral solution: *MEKINIST for oral solution should only be given by a caregiver.
| |
|
What are the possible side effects of MEKINIST? *See “What is the most important information I should know about MEKINIST?” *bleeding problems. MEKINIST can cause serious bleeding problems, especially in your brain or stomach, that can lead to death. Call your healthcare provider and get medical help right away if you have any signs of bleeding, including: *inflammation of the intestines, or tears (perforation) of the stomach or intestines. MEKINIST can cause inflammation of your intestines, or tears in the stomach or intestines that can lead to death. Tell your healthcare provider right away if you have any of the following symptoms: *blood clots. MEKINIST can cause blood clots in your arms or legs, which can travel to your lungs and can lead to death. Get medical help right away if you have the following symptoms: *heart problems, including heart failure. Your healthcare provider should check your heart function before and during treatment with MEKINIST. Call your healthcare provider right away if you have any of the following signs and symptoms of a heart problem: *eye problems. MEKINIST can cause severe eye problems that might lead to blindness. Call your healthcare provider right away if you get these symptoms of eye problems: *lung or breathing problems. MEKINIST can cause lung or breathing problems. Tell your healthcare provider if you have any new or worsening symptoms of lung or breathing problems, including: *fever. Fever is common during treatment with MEKINIST and dabrafenib, but it may also be serious. When taking MEKINIST with dabrafenib, fever may happen more often or may be more severe. In some cases, chills or shaking chills, too much fluid loss (dehydration), low blood pressure, dizziness, or kidney problems may happen with the fever. *serious skin reactions. Skin rash is a common side effect of MEKINIST. MEKINIST can also cause other skin reactions. In some cases, these rashes and other skin reactions can be severe or serious and may need to be treated in a hospital or lead to death. Tell your healthcare provider if you get a skin rash or acne that bothers you or worsens. Tell your healthcare provider right away if you develop any of the following signs or symptoms of a severe skin reaction, including: | |
|
○ blisters or peeling of your skin |
○ blisters on your lips, or around your mouth or eyes |
|
*increased blood sugar (hyperglycemia). Some people may develop high blood sugar or worsening diabetes during treatment with MEKINIST and dabrafenib. If you are diabetic, your healthcare provider should check your blood sugar levels closely during treatment with MEKINIST and dabrafenib. Your diabetes medicine may need to be changed. Tell your healthcare provider if you have any of the following symptoms of severe high blood sugar: *hemophagocytic lymphohistiocytosis (HLH). MEKINIST when taken or given with dabrafenib may increase the risk of a type of overactivity of the immune system (hemophagocytic lymphohistiocytosis) that can cause fever, swollen glands, bruising, or skin rash. If you experience a combination of these symptoms, call your healthcare provider right away. The most common side effects of MEKINIST when taken alone include:
| |
|
The most common side effects of MEKINIST when taken with dabrafenib in people with melanoma that has spread to other parts of the body or cannot be removed by surgery include: | |
|
• fever |
• diarrhea |
|
• rash |
• vomiting |
|
• nausea |
• high blood pressure (hypertension) |
|
• chills |
• swelling of the face, arms, or legs |
|
The most common side effects of MEKINIST when taken with dabrafenib to help prevent melanoma from coming back after the cancer has been removed by surgery include: | |
|
• fever |
• chills |
|
• tiredness |
• diarrhea |
|
• nausea |
• vomiting |
|
• headache |
• joint aches |
|
• rash |
• muscle aches |
|
The most common side effects of MEKINIST when taken with dabrafenib in people with NSCLC include: | |
|
• fever |
• rash |
|
• tiredness |
• swelling of your face, arms, and legs |
|
• nausea |
• chills |
|
• vomiting |
• bleeding |
|
• diarrhea |
• cough |
|
• dry skin |
• shortness of breath |
|
• decreased appetite | |
|
The most common side effects of MEKINIST when taken with dabrafenib in adults with solid tumors that cannot be removed by surgery or have spread to other parts of the body include: | |
|
• fever |
• bleeding |
|
• tiredness |
• cough |
|
• nausea |
• vomiting |
|
• rash |
• constipation |
|
• chills |
• diarrhea |
|
• headache |
• muscle and joint aches |
|
• swelling of your arms and legs | |
|
The most common side effects of MEKINIST when given with dabrafenib in children 1 year of age and older with solid tumors that cannot be removed by surgery or have spread to other parts of the body include: | |
|
• fever |
• acne |
|
• rash |
• headache |
|
• vomiting |
• stomach-area (abdominal) pain |
|
• tiredness |
• nausea |
|
• dry skin |
• bleeding |
|
• cough |
• constipation |
|
• diarrhea |
• skin infection around fingernails or toenails |
|
The most common side effects of MEKINIST when given with dabrafenib in children 1 year of age and older with low-grade glioma include: | |
|
• fever |
• dry skin |
|
• rash |
• diarrhea |
|
• headache |
• nausea |
|
• vomiting |
• bleeding |
|
• muscle and bone pain |
• stomach-area (abdominal) pain |
|
• tiredness |
• acne |
|
MEKINIST can cause new or worsening high blood pressure (hypertension).
Your healthcare provider should check your blood pressure during treatment
with MEKINIST. Call your healthcare provider right away if you develop high
blood pressure, your blood pressure worsens, or you have severe headache,
lightheadedness, blurry vision, or dizziness. | |
|
How should I store MEKINIST?
MEKINIST for oral solution:
Keep MEKINIST and all medicines out of the reach of children. | |
|
General information about the safe and effective use of MEKINIST | |
|
What are the ingredients in MEKINIST? Distributed by: Novartis Pharmaceuticals Corporation, East Hanover, New Jersey 07936 © Novartis For more information, go to www.us.tafinlarmekinist.com or call 1-888-669-6682. |
T2024-26
CLINICAL STUDIES SECTION
14** CLINICAL STUDIES**
14.1 BRAF V600E or V600K Mutation-Positive Unresectable or Metastatic
Melanoma
MEKINIST as a Single Agent
The safety and efficacy of MEKINIST were evaluated in an international, multi- center, randomized (2:1), open-label, active-controlled trial (the METRIC study; NCT01245062) in 322 patients with BRAF V600E or V600K mutation- positive, unresectable or metastatic melanoma. In the METRIC study, patients were not permitted to have more than one prior chemotherapy regimen for advanced or metastatic disease; prior treatment with a BRAF inhibitor or MEK inhibitor was not permitted. Patients were randomized to receive MEKINIST 2 mg orally once daily (N = 214) or chemotherapy (N = 108) consisting of either dacarbazine 1000 mg/m2 intravenously every 3 weeks or paclitaxel 175 mg/m2 intravenously every 3 weeks. Treatment continued until disease progression or unacceptable toxicity. Randomization was stratified according to prior use of chemotherapy for advanced or metastatic disease (yes vs. no) and LDH level (normal vs. greater than ULN). Tumor tissue was evaluated for BRAF mutations at a central testing site using a clinical trial assay. Tumor samples from 289 patients (196 patients treated with MEKINIST and 93 chemotherapy-treated patients) were also tested retrospectively using an FDA-approved companion diagnostic test, THxID®-BRAF assay. The major efficacy outcome measure was progression-free survival (PFS).
The median age for randomized patients was 54 years, 54% were male, greater than 99% were White, and all patients had baseline ECOG performance status of 0 or 1. Most patients had metastatic disease (94%), had M1c disease (64%), had elevated LDH (36%), had no history of brain metastasis (97%), and received no prior chemotherapy for advanced or metastatic disease (66%). The distribution of BRAF V600 mutations was BRAF V600E (87%), V600K (12%), or both (less than 1%). The median durations of follow-up prior to initiation of alternative treatment were 4.9 months for patients treated with MEKINIST and 3.1 months for patients treated with chemotherapy. Fifty-one (47%) patients crossed over from the chemotherapy arm at the time of disease progression to receive MEKINIST.
The METRIC study demonstrated a statistically significant increase in PFS in the patients treated with MEKINIST. Table 20 and Figure 1 summarize the PFS results.
Table 20. Efficacy Results in the METRIC Study|
Abbreviations: CI, confidence interval; DoR, duration of response; HR, hazard
ratio; NR, not reached. | ||
|
Investigator**-Assessed Endpoints** |
MEKINIST |
Chemotherapy |
|
Progression-FreeS****urvival | ||
|
Number of events (%) |
117 (55%) |
77 (71%) |
|
Progressive disease |
107 (50%) |
70 (65%) |
|
Death |
10 (5%) |
7 (6%) |
|
Median, months (95% CI) |
4.8 (4.3, 4.9) |
1.5 (1.4, 2.7) |
|
HRa (95% CI) |
0.47 (0.34, 0.65) | |
|
P value (log-rank test) |
< 0.0001 | |
|
Confirmed Tumor Responses | ||
|
Overall response rate (95% CI) |
22% (17%, 28%) |
8% (4%, 15%) |
|
Complete response, n (%) |
4 (2%) |
0 |
|
Partial response, n (%) |
43 (20%) |
9 (8%) |
|
Duration of Response | ||
|
Median DoR, months (95% CI) |
5.5 (4.1, 5.9) |
NR (3.5, NR) |
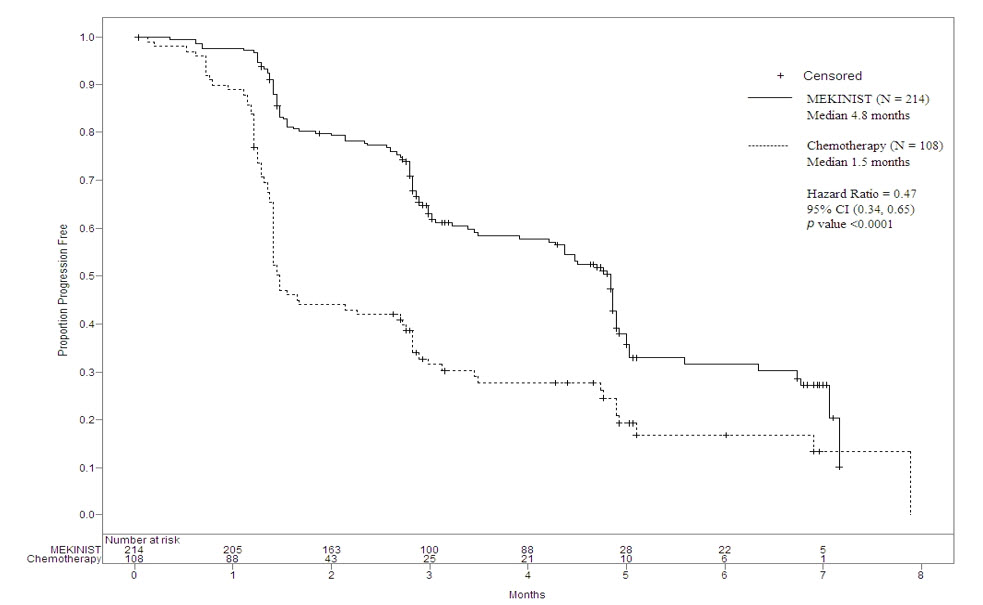
Figure 1. Kaplan-Meier Curves of Investigator-Assessed Progression-Free Survival (ITT Population) in the METRIC Study
In supportive analyses based on independent radiologic review committee (IRRC) assessment, the PFS results were consistent with those of the primary efficacy analysis.
MEKINIST with Dabrafenib
COMBI-d Study
The safety and efficacy of MEKINIST administered with dabrafenib were evaluated in an international, randomized, double-blind, active-controlled trial (the COMBI-d study; NCT01584648). The COMBI-d study compared dabrafenib plus MEKINIST to dabrafenib plus placebo as first-line treatment for patients with unresectable (Stage IIIC) or metastatic (Stage IV) BRAF V600E or V600K mutation-positive cutaneous melanoma. Patients were randomized (1:1) to receive MEKINIST 2 mg once daily plus dabrafenib 150 mg twice daily or dabrafenib 150 mg twice daily plus matching placebo. Randomization was stratified by LDH level (> ULN vs. ≤ ULN) and BRAF mutation subtype (V600E vs. V600K). The major efficacy outcome was investigator-assessed PFS per RECIST v1.1 with additional efficacy outcome measures of overall survival (OS) and confirmed overall response rate (ORR).
In the COMBI-d study, 423 patients were randomized to MEKINIST plus dabrafenib (n = 211) or dabrafenib plus placebo (n = 212). The median age was 56 years (range: 22 to 89), 53% were male, > 99% were White, 72% had ECOG performance status of 0, 4% had Stage IIIC, 66% had M1c disease, 65% had normal LDH, and 2 patients had a history of brain metastases. All patients had tumor containing BRAF V600E or V600K mutations as determined by centralized testing with the FDA-approved companion diagnostic test; 85% had BRAF V600E mutation-positive melanoma and 15% had BRAF V600K mutation-positive melanoma.
The COMBI-d study demonstrated statistically significant improvements in PFS and OS. Table 21 and Figure 2 summarize the efficacy results.
Table 21. Efficacy Results in the COMBI-d Study|
Abbreviations: CI, confidence interval; DoR, duration of response; HR, hazard
ratio; NR, not reached; ORR, overall response rate. | ||
|
Endpoint |
MEKINISTplusD****abrafenib |
Placebo plus Dabrafenib |
|
Progression-Free Survival****a | ||
|
Number of events (%) |
102 (48%) |
109 (51%) |
|
Median, months (95% CI) |
9.3 (7.7, 11.1) |
8.8 (5.9, 10.9) |
|
HR (95% CI) |
0.75 (0.57, 0.99) | |
|
P valueb |
0.035 | |
|
Overall Survival | ||
|
Number of deaths (%) |
99 (47%) |
123 (58%) |
|
Median, months (95% CI) |
25.1 (19.2, NR) |
18.7 (15.2, 23.1) |
|
HR (95% CI) |
0.71 (0.55, 0.92) | |
|
P valueb |
0.01 | |
|
Overall Response Rate****a | ||
|
ORR (95% CI) |
66% (60%, 73%) |
51% (44%, 58%) |
|
P value |
< 0.001 | |
|
Complete response |
10% |
8% |
|
Partial response |
56% |
42% |
|
Median DoR, months (95% CI) |
9.2 (7.4, NR) |
10.2 (7.5, NR) |
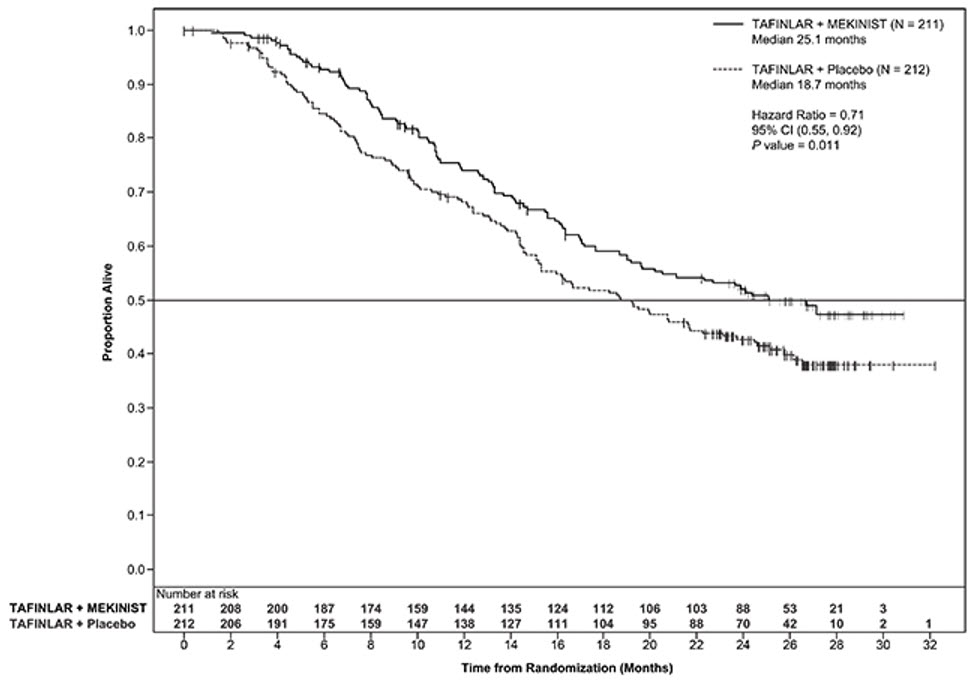
Figure 2. Kaplan-Meier Curves of Overall Survival in the COMBI-d Study
COMBI-MB Study
The activity of MEKINIST with dabrafenib for the treatment of BRAF V600E or V600K mutation-positive melanoma, metastatic to the brain, was evaluated in a non-randomized, open-label, multi-center, multi-cohort trial (the COMBI-MB study; NCT02039947). Eligible patients were required to have at least one measurable intracranial lesion and to have no leptomeningeal disease, parenchymal brain metastasis greater than 4 cm in diameter, ocular melanoma, or primary mucosal melanoma. Patients received MEKINIST 2 mg orally once daily and dabrafenib 150 mg orally twice daily until disease progression or unacceptable toxicity. The major efficacy outcome measure was intracranial response rate, defined as the percentage of patients with a confirmed intracranial response per RECIST v1.1, modified to allow up to five intracranial target lesions at least 5 mm in diameter, as assessed by independent review.
The COMBI-MB study enrolled 121 patients with a BRAF V600E (85%) or V600K (15%) mutation. The median age was 54 years (range: 23 to 84), 58% were male, 100% were White, 8% were from the United States, 65% had normal LDH at baseline, and 97% had an ECOG performance status of 0 or 1. Intracranial metastases were asymptomatic in 87% and symptomatic in 13% of patients, 22% received prior local therapy for brain metastases, and 87% also had extracranial metastases.
The intracranial response rate was 50% (95% CI: 40, 60), with a complete response rate of 4.1% and a partial response rate of 46%. The median duration of intracranial response was 6.4 months (range: 1 to 31). Of the patients with an intracranial response, 9% had stable or progressive disease as their best overall response.
14.2 Adjuvant Treatment of BRAF V600E or V600K Mutation-Positive Melanoma
The safety and efficacy of MEKINIST administered with dabrafenib were evaluated in an international, multi-center, randomized, double-blind, placebo-controlled trial (COMBI-AD; NCT01682083) that enrolled patients with Stage III melanoma with BRAF V600E or V600K mutations as detected by the THxID®-BRAF assay and pathologic involvement of regional lymph node(s). Enrollment required complete resection of melanoma with complete lymphadenectomy within 12 weeks prior to randomization. The trial excluded patients with mucosal or ocular melanoma, unresectable in-transit metastases, distant metastatic disease, or prior systemic anti-cancer treatment, including radiotherapy. Patients were randomized (1:1) to receive MEKINIST 2 mg once daily in combination with dabrafenib 150 mg twice daily or two placebos for up to 1 year. Randomization was stratified by BRAF mutation status (V600E or V600K) and American Joint Committee on Cancer (AJCC; 7th Edition) Stage (IIIA, IIIB, or IIIC). The major efficacy outcome measure was relapse-free survival (RFS) defined as the time from randomization to disease recurrence (local, regional, or distant metastasis), new primary melanoma, or death from any cause, whichever occurred first as assessed by the investigator. Patients underwent imaging for tumor recurrence every 3 months for the first two years and every 6 months thereafter.
In COMBI-AD, a total of 870 patients were randomized: 438 to the MEKINIST in combination with dabrafenib and 432 to placebo. Median age was 51 years (range: 18 to 89), 55% were male, 99% were White, and 91% had an ECOG performance status of 0. Disease characteristics were AJCC Stage IIIA (18%), Stage IIIB (41%), Stage IIIC (40%), stage unknown (1%); BRAF V600E mutation (91%), BRAF V600K mutation (9%); macroscopic lymph nodes (65%); and tumor ulceration (41%). The median duration of follow-up (time from randomization to last contact or death) was 2.8 years.
COMBI-AD showed a statistically significant improvement in RFS in patients randomized to MEKINIST in combination with dabrafenib arm compared to those randomized to placebo. Efficacy results are presented in Table 22 and Figure 3.
Table 22. Efficacy Results in COMBI-AD in the Adjuvant Treatment of Melanoma|
Abbreviations: HR, hazard ratio; CI, confidence interval; NE, not estimable. | ||
|
Endpoint |
MEKINIST plus Dabrafenib |
Placebo |
|
Relapse-Free Survival | ||
|
Number of events (%) |
166 (38) |
248 (57) |
|
Median, months (95% CI) |
NE (44.5, NE) |
16.6 (12.7, 22.1) |
|
HR (95% CI)a |
0.47 (0.39, 0.58) | |
|
P valueb |
< 0.0001 |
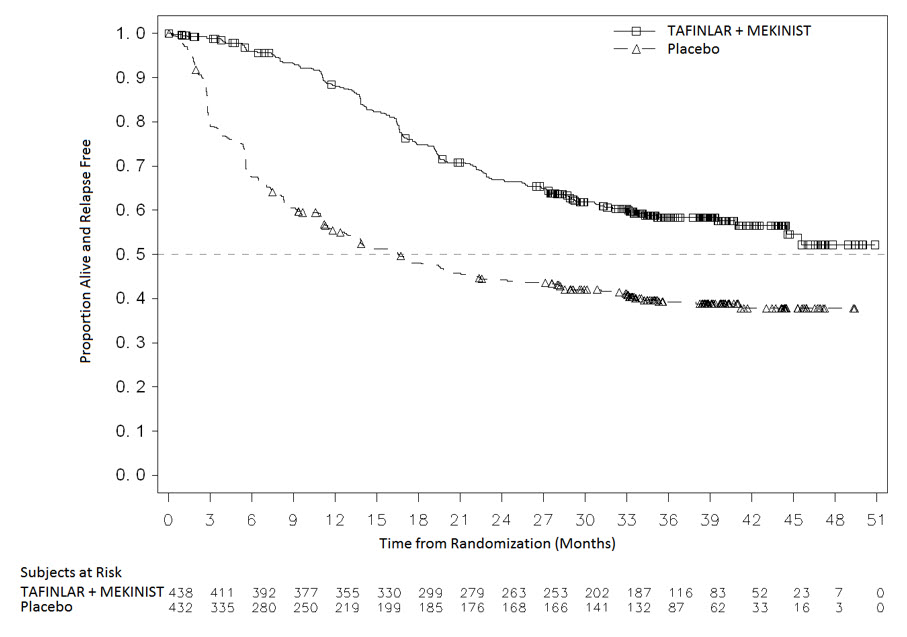
Figure 3. Kaplan-Meier Curves for Relapse-Free Survival in COMBI-AD in the Adjuvant Treatment of Melanoma
14.3 BRAF V600E Mutation-Positive Metastatic Non-Small Cell Lung Cancer
The safety and efficacy of dabrafenib alone or administered with MEKINIST were evaluated in a multi-center, three-cohort, non-randomized, activity- estimating, open-label trial (Study BRF113928; NCT01336634). Key eligibility criteria were locally confirmed BRAF V600E mutation-positive metastatic NSCLC, no prior exposure to BRAF or MEK inhibitor, and absence of EGFR mutation or ALK rearrangement (unless patients had progression on prior tyrosine kinase inhibitor therapy). Patients enrolled in Cohorts A and B were required to have received at least one previous platinum-based chemotherapy regimen with demonstrated disease progression but no more than three prior systemic regimens. Patients in Cohort C could not have received prior systemic therapy for metastatic disease. Patients in Cohort A received dabrafenib 150 mg twice daily. Patients in Cohorts B and C received MEKINIST 2 mg once daily and dabrafenib 150 mg twice daily. The major efficacy outcome was ORR per RECIST v1.1 as assessed by independent review committee (IRC) and duration of response.
There were a total of 171 patients enrolled which included 78 patients enrolled in Cohort A, 57 patients enrolled in Cohort B, and 36 patients enrolled in Cohort C. The characteristics of the population were: a median age of 66 years; 48% male; 81% White, 14% Asian, 3% Black, and 2% Hispanic; 60% former smokers, 32% never smokers, and 8% current smokers; 27% had ECOG performance status (PS) of 0, 63% had ECOG PS of 1, and 11% had ECOG PS of 2; 99% had metastatic disease of which 6% had brain metastasis at baseline and 14% had liver metastasis at baseline; 11% had systemic anti-cancer therapy in the adjuvant setting, 58% of the 135 previously treated patients had only one line of prior systemic therapy for metastatic disease; 98% had non-squamous histology.
Efficacy results are summarized in Table 23.
Table 23. Efficacy Results Based on Independent Review in Study BRF113928|
Abbreviations: CI, confidence interval; DoR, duration of response. | |||
|
Treatment |
Dabrafenib |
MEKINIST plus Dabrafenib | |
|
Population |
Previously Treated |
Previously Treated |
Treatment Naïve |
|
Overall Response Rate****a | |||
|
ORR (95% CI) |
27% (18%, 38%) |
61% (48%, 74%) |
61% (44%, 77%) |
|
Complete response |
1% |
5% |
8% |
|
Partial response |
26% |
56% |
53% |
|
Duration of Response****a |
n = 21 |
n = 35 |
n = 22 |
|
Median DoR, months (95% CI) |
18.0 (4.2, 40.1) |
9.0 (5.8, 26.2) |
15.2 (7.8, 23.5) |
In a subgroup analysis of patients with retrospectively centrally confirmed BRAF V600E mutation-positive NSCLC with the Oncomine™ Dx Target Test, the ORR results were similar to those presented in Table 16.
14.4 BRAF V600E Mutation-Positive Locally Advanced or Metastatic Anaplastic
Thyroid Cancer
The safety and efficacy of MEKINIST administered with dabrafenib was evaluated in an activity-estimating, nine-cohort, multi-center, non-randomized, open- label trial (Study BRF117019; NCT02034110) in patients with rare cancers with the BRAF V600E mutation, including locally advanced, unresectable, or metastatic ATC with no standard locoregional treatment options. Trial BRF117019 excluded patients who could not swallow or retain the medication; who received prior treatment with BRAF or MEK inhibitors; with symptomatic or untreated CNS metastases; or who had airway obstruction. Patients received MEKINIST 2 mg once daily and dabrafenib 150 mg twice daily. The major efficacy outcome measure was ORR per RECIST v1.1 as assessed by independent review committee (IRC) and duration of response (DoR).
Thirty-six patients were enrolled and were evaluable for response in the ATC cohort. The median age was 71 years (range: 47 to 85); 44% were male, 50% White, 44% Asian; and 94% had ECOG performance status of 0 or 1. Prior anti- cancer treatments included surgery and external beam radiotherapy (83% each), and systemic therapy (67%).
Efficacy results are summarized in Table 24.
Table 24. Efficacy Results in the ATC Cohort Based on Independent Review of Study BRF117019|
Abbreviations: ATC, anaplastic thyroid cancer; CI, confidence interval; DoR, duration of response; ORR, overall response rate; NE, not estimable. | |
|
ATC Cohort Population |
N = 36 |
|
Overall Response Rate | |
|
ORR (95% CI) |
53% (35.5%, 69.6%) |
|
Complete response |
6% |
|
Partial response |
47% |
|
Duration of Response |
n = 19 |
|
Median DoR, months (95% CI) |
13.6 (3.8, NE) |
|
% with DoR ≥ 6 months |
68% |
|
% with DoR ≥ 12 months |
53% |
14.5 Lack of Clinical Activity in Metastatic Melanoma Following BRAF-
Inhibitor Therapy
The clinical activity of MEKINIST as a single agent was evaluated in a single- arm, multi-center, international trial in 40 patients with BRAF V600E or V600K mutation-positive, unresectable or metastatic melanoma who had received prior treatment with a BRAF inhibitor. All patients received MEKINIST at a dose of 2 mg orally once daily until disease progression or unacceptable toxicity.
The median age was 58 years, 63% were male, all were White, 98% had baseline ECOG PS of 0 or 1, and the distribution of BRAF V600 mutations was V600E (83%), V600K (10%), and the remaining patients had multiple V600 mutations (5%), or unknown mutational status (2%). No patient achieved a confirmed partial or complete response as determined by the clinical investigators.
14.6 BRAF V600E Mutation-Positive Unresectable or Metastatic Solid Tumors
The safety and efficacy of MEKINIST in combination with dabrafenib for the treatment of BRAF V600E mutation-positive unresectable or metastatic solid tumors were evaluated in Trials BRF117019, NCI-MATCH, and CTMT212X2101, and supported by results in COMBI-d, COMBI-v [see Clinical Studies (14.2)], and BRF113928 [see Clinical Studies (14.4)]. In adult studies, patients received MEKINIST 2 mg once daily and dabrafenib 150 mg twice daily. The major efficacy outcome measures were ORR per RECIST v1.1, RANO [HGG] or modified RANO [LGG] criteria and duration of response (DoR).
BRF117019 Study and NCI-MATCH Study
Study BRF117019 (NCT02034110) [see Clinical Studies (14.5)] is a multi-cohort, multi-center, non-randomized, open-label trial in adult patients with selected tumors with the BRAF V600E mutation, including high grade glioma (HGG) (n = 45), biliary tract cancer (BTC) (n = 43), low grade glioma (LGG) (n = 13), adenocarcinoma of small intestine (ASI) (n = 3), gastrointestinal stromal tumor (GIST) (n = 1), and anaplastic thyroid cancer [see Clinical Studies (14.5)]. Patients were enrolled based on local assessments of BRAF V600E mutation status; a central laboratory confirmed the BRAF V600E mutation in 93 of 105 patients.
Arm H (EAY131-H) of the NCI-MATCH study (NCT02465060) is a single-arm, open- label study that enrolled patients with a BRAF V600E mutation. Patients with melanoma, thyroid cancer, or CRC were excluded. BRAF mutation status for enrollment was determined either by central or local laboratory test. The study included adult patients with solid tumors including gastrointestinal tumors (n = 14), lung tumors (n = 7), gynecologic or peritoneal tumors (n = 6), CNS tumors (n = 4), and ameloblastoma of mandible (n = 1).
Among the 131 patients enrolled in BRF117019 and NCI-MATCH with the tumor types shown in Table 21, the baseline characteristics were: median age of 51 years with 20% age 65 or older; 56% female; 85% White, 9% Asian, 3% Black, 3% other; and 37% ECOG 0, 56% ECOG 1, and 6% ECOG 2. Of the 131 patients, 90% received prior systemic therapy.
Efficacy results in patients with solid tumors are summarized in Table 25.
Table 25. Efficacy Results Based on Independent Review in Study BRF117019 and NCI-MATCH Arm H|
Abbreviations: PR, partial response. | ||||
|
Tumor Type****a |
N |
Objective Response Rate |
Duration of Response | |
|
% |
95% CI |
Range (months) | ||
|
Biliary tract cancerb |
48 |
46 |
(31, 61) |
1.8d, 40d |
|
High grade gliomac |
48 |
33 |
(20, 48) |
3.9, 44 |
|
Glioblastoma |
32 |
25 |
(12, 43) |
3.9, 27 |
|
Anaplastic pleomorphic xanthoastrocytoma |
6 |
67 |
(22, 96) |
6, 43 |
|
Anaplastic astrocytoma |
5 |
20 |
(0.5, 72) |
15 |
|
Astroblastoma |
2 |
100 |
(16, 100) |
15, 23d |
|
Undifferentiated |
1 |
PR |
(2.5, 100) |
6 |
|
Anaplastic ganglioglioma |
1 |
0 |
NA |
NA |
|
Anaplastic oligodendroglioma |
1 |
0 |
NA |
NA |
|
Low grade glioma |
14 |
50 |
(23, 77) |
6, 29d |
|
Astrocytoma |
4 |
50 |
(7, 93) |
7, 23 |
|
Ganglioglioma |
4 |
50 |
(7, 93) |
6, 13 |
|
Pleomorphic xanthoastrocytoma |
2 |
50 |
(1.3, 99) |
6 |
|
Pilocytic astrocytoma |
2 |
0 |
NA |
NA |
|
Choroid plexus papilloma |
1 |
PR |
(2.5, 100) |
29d |
|
Gangliocytoma/ganglioglioma |
1 |
PR |
(2.5, 100) |
18d |
|
Low grade serous ovarian carcinoma |
5 |
80 |
(28, 100) |
12, 42d |
|
Adenocarcinoma small intestine |
4 |
50 |
(7, 93) |
7, 8 |
|
Adenocarcinoma pancreas |
3 |
0 |
NA |
NA |
|
Mixed ductal/adenoneuroendocrine carcinoma |
2 |
0 |
NA |
NA |
|
Neuroendocrine carcinoma of colon |
2 |
0 |
NA |
NA |
|
Ameloblastoma of mandible |
1 |
PR |
(2.5, 100) |
30 |
|
Combined small cell-squamous carcinoma of lung |
1 |
PR |
(2.5, 100) |
5 |
|
Mucinous-papillary serous adenocarcinoma of peritoneum |
1 |
PR |
(2.5, 100) |
8 |
|
Adenocarcinoma of anus |
1 |
0 |
NA |
NA |
|
Gastrointestinal stromal tumor |
1 |
0 |
NA |
NA |
CTMT212X2101 (X2101) Study
Study X2101 (NCT02124772) was a multi-center, open-label, multi-cohort study in pediatric patients with refractory or recurrent solid tumors. Part C was a dose escalation of MEKINIST in combination with dabrafenib in patients with a BRAF V600E mutation. Part D was a cohort expansion phase of MEKINIST in combination with dabrafenib in patients with LGG with a BRAF V600E mutation. The major efficacy outcome measure was ORR as assessed by independent review committee per RANO criteria.
The efficacy of MEKINIST in combination with dabrafenib was evaluated in 48 pediatric patients, including 34 patients with LGG and 2 patients with HGG.
For patients with BRAF V600E mutant LGG and HGG in Parts C and D, the median age was 10 years (range: 1 to 17); 50% were male, 75% White, 8% Asian, 3% Black; and 58% had Karnofsky/Lansky performance status of 100. Prior anti- cancer treatments included surgery (83%), external beam radiotherapy (2.8%), and systemic therapy (92%). The ORR was 25% (95% CI: 12%, 42%). For the 9 patients who responded, DoR was ≥ 6 months for 78% of patients and ≥ 24 months for 44% of patients.
CDRB436G2201 (G2201) Study – High-Grade Glioma Cohort
Study G2201 (NCT02684058) was a multi-center, randomized, open-label, Phase II study of dabrafenib and trametinib in chemotherapy naïve pediatric patients with BRAF V600E mutant low-grade glioma (LGG) and patients with relapsed or progressive BRAF V600E mutant HGG. Patients with HGG were enrolled in a single-arm cohort. The major efficacy outcome measure for the HGG cohort was ORR as assessed by independent review committee per RANO 2010 criteria.
The efficacy of MEKINIST in combination with dabrafenib was evaluated in 41 pediatric patients with relapsed or progressive HGG.
For patients with BRAF V600E mutant HGG enrolled in the HGG cohort, the median age was 13 years (range: 2 to 17); 56% were female, 61% White, 27% Asian, 2.4% Black, and 37% had Karnofsky/Lansky performance status of 100. Prior anti- cancer treatments included surgery (98%), radiotherapy (90%), and chemotherapy (81%). The ORR was 56% (95% CI: 40, 72). The median DoR was not reached (95% CI: 9.2, NE). For the 23 patients who responded in the HGG cohort, DoR was ≥ 6 months for 78% of patients, ≥ 12 months for 48% of patients, and ≥ 24 months for 22% of patients.
14.7 BRAF V600E Mutation-Positive Low-Grade Glioma
CDRB436G2201 (G2201) Study – Low-Grade Glioma Cohort
The safety and efficacy of MEKINIST in combination with dabrafenib for the treatment of BRAF V600E mutation-positive low-grade glioma (LGG) in pediatric patients aged 1 to < 18 years of age were evaluated in the multi-center, open- label trial (Study CDRB436G2201; NCT02684058). Patients with LGG (WHO grades 1 and 2) who required first systemic therapy were randomized in a 2:1 ratio to dabrafenib plus trametinib (D + T) or carboplatin plus vincristine (C + V).
BRAF mutation status was identified prospectively via a local assessment or a central laboratory test. In addition, retrospective testing of available tumor samples by the central laboratory was performed to evaluate BRAF V600E mutation status.
Patients received age- and weight-based dosing of MEKINIST and dabrafenib until loss of clinical benefit or until unacceptable toxicity. Carboplatin and vincristine were dosed based on body surface area at doses 175 mg/m2 and 1.5 mg/m2 (0.05 mg/kg for patients < 12 kg), respectively, as one 10-week induction course followed by eight 6-week cycles of maintenance therapy.
The major efficacy outcome measure was overall response rate (ORR) by independent review based on RANO LGG (2017) criteria. Additional efficacy outcome measures were progression-free survival and overall survival. The primary analysis was performed when all patients had completed at least 32 weeks of therapy.
In the LGG cohort, 110 patients were randomized to D + T (n = 73) or C + V (n = 37). Median age was 9.5 years (range: 1 to 17); 60% were female. Study G2201 showed a statistically significant improvement in ORR and PFS in patients with LGG randomized to D + T compared to those randomized to C + V. Efficacy results are shown in Table 26.
Table 26. Efficacy Results Based on Independent Review in Study G2201 (LGG cohort)|
Abbreviations: CI, confidence interval; NE, not estimable. | ||
|
MEKINIST plus Dabrafenib |
Carboplatin plus Vincristine | |
|
Overall Response Rate | ||
|
ORR% (95% CI)a |
46.6 (34.8, 58.6) |
10.8 (3.0, 25.4) |
|
P value |
< 0.001 | |
|
Complete response, n (%) |
2 (2.7) |
1 (2.7) |
|
Partial response, n (%) |
32 (44) |
3 (8) |
|
Duration of Response | ||
|
Median (95% CI)b, months |
23.7 (14.5, NE) |
NE (6.6, NE) |
|
% with observed DoR ≥ 12 months |
56 |
50 |
|
% with observed DoR ≥ 24 months |
15 |
25 |
|
Progression-Free Survival | ||
|
Median (95% CI)b, months |
20.1 (12.8, NE) |
7.4 (3.6, 11.8) |
|
Hazard ratio (95% CI)c |
0.31 (0.17, 0.55) | |
|
P value |
< 0.001 |
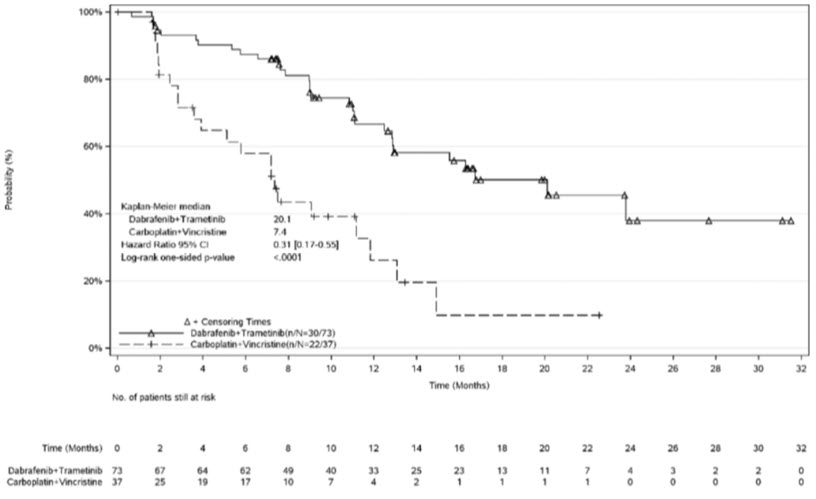
Figure 4. Kaplan-Meier Curves for Progression-Free Survival in Study G2201 (LGG cohort)
At the time of the interim analysis of overall survival (OS), conducted when all patients had completed at least 32 weeks of treatment or had discontinued earlier, there was one death on the C + V arm. The OS results at interim analysis did not reach statistical significance.
DESCRIPTION SECTION
11** DESCRIPTION**
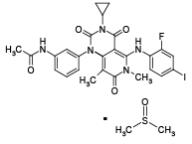
Trametinib dimethyl sulfoxide is a kinase inhibitor. The chemical name is acetamide, N-[3-[3-cyclopropyl-5-[(2-fluoro-4- iodophenyl)amino]-3,4,6,7-tetrahydro-6,8-dimethyl- 2,4,7-trioxopyrido[4,3-d]pyrimidin-1(2H)-yl]phenyl]-, compound with 1,1’-sulfinylbis[methane] (1:1). It has a molecular formula C26H23FIN5O4•C2H6OS with a molecular mass of 693.53 g/mol. Trametinib dimethyl sulfoxide has the following chemical structure:
Trametinib dimethyl sulfoxide is a white to almost white powder. It is practically insoluble in the pH range of 2 to 8 in aqueous media.
MEKINIST (trametinib) tablets for oral use are supplied as 0.5 mg and 2 mg tablets for oral administration. Each 0.5 mg tablet contains 0.5635 mg trametinib dimethyl sulfoxide equivalent to 0.5 mg of trametinib non-solvated parent. Each 2 mg tablet contains 2.254 mg trametinib dimethyl sulfoxide equivalent to 2 mg of trametinib non-solvated parent.
The inactive ingredients of MEKINIST tablets are: Tablet Core: colloidal silicon dioxide, croscarmellose sodium, hypromellose, magnesium stearate (vegetable source), mannitol, microcrystalline cellulose, and sodium lauryl sulfate. Coating: hypromellose, iron oxide red (2 mg tablets), iron oxide yellow (0.5 mg tablets), polyethylene glycol, polysorbate 80 (2 mg tablets), and titanium dioxide.
MEKINIST (trametinib) for oral solution is a white or almost white powder which produces a clear colorless solution when reconstituted with water. Each bottle contains 4.7 mg of trametinib equivalent to 5.3 mg trametinib dimethyl sulfoxide. Each mL of reconstituted trametinib solution contains 0.05 mg of trametinib non-solvated parent. The inactive ingredients of MEKINIST for oral solution are betadex sulfobutyl ether sodium, citric acid monohydrate, dibasic sodium phosphate, methylparaben, potassium sorbate, sucralose, and strawberry flavor.
CLINICAL PHARMACOLOGY SECTION
12** CLINICAL PHARMACOLOGY**
12.1 Mechanism of Action
Trametinib is a reversible inhibitor of mitogen-activated extracellular signal-regulated kinase 1 (MEK1) and MEK2 activation and of MEK1 and MEK2 kinase activity. MEK proteins are upstream regulators of the extracellular signal-related kinase (ERK) pathway, which promotes cellular proliferation. BRAF V600E mutations result in constitutive activation of the BRAF pathway which includes MEK1 and MEK2. Trametinib inhibits cell growth of various BRAF V600 mutation-positive tumors in vitro and in vivo.
Trametinib and dabrafenib target two different kinases in the RAS/RAF/MEK/ERK pathway. Use of trametinib and dabrafenib in combination resulted in greater growth inhibition of BRAF V600 mutation-positive tumor cell lines in vitro and prolonged inhibition of tumor growth in BRAF V600 mutation-positive tumor xenografts compared with either drug alone.
In the setting of BRAF-mutant colorectal cancer, induction of EGFR-mediated MAPK pathway re-activation has been identified as a mechanism of intrinsic resistance to BRAF inhibitors [see Indications and Usage (1.7)].
12.2 Pharmacodynamics
Administration of MEKINIST tablets 1 mg and 2 mg to patients with BRAF V600 mutation-positive melanoma resulted in dose-dependent changes in tumor biomarkers, including inhibition of phosphorylated ERK, inhibition of Ki67 (a marker of cell proliferation), and increases in p27 (a marker of apoptosis).
Cardiac Electrophysiology
The heart rate-corrected QT (QTc) prolongation potential of trametinib was assessed in a dedicated study in 32 patients who received placebo on Day 1 and MEKINIST tablets 2 mg once daily on Days 2-14 followed by MEKINIST tablets 3 mg on Day 15. No large changes in the mean QTc interval (i.e., > 20 ms) were detected in the study.
A decrease from baseline in HR by 9 beats/min (90% CI: -11.4 to -6.1) and an increase from baseline in PR by 20 ms (90% CI: 13.0 to 27.4) relative to placebo was observed at two hours post-dose in the same study.
In clinical trials in patients who received MEKINIST with dabrafenib, QTc prolongation > 500 ms occurred in 0.8% of patients and QTc increased by > 60 ms from baseline in 3.8% of patients.
12.3 Pharmacokinetics
The pharmacokinetics of trametinib were characterized following a single dose and multiple doses in patients with solid tumors and BRAF V600 mutation- positive metastatic melanoma. Following administration of MEKINIST tablets 0.125 mg (0.0625 times the approved recommended adult dosage) to 4 mg (2 times the approved recommended adult dosage) daily, both Cmax and AUC increase proportionally with dose. Inter-subject variability in AUC and Cmax at steady state is 22% and 28%, respectively.
Absorption
The median time to achieve peak plasma concentrations (Tmax) is 1.5 hours post-dose. The mean absolute bioavailability of MEKINIST tablets is 72% and MEKINIST for oral solution is 81%.
Effect of Food
Following administration of MEKINIST tablets, a high-fat, high-calorie meal (approximately 1000 calories) decreased trametinib AUC by 24%, Cmax by 70%, and delayed Tmax by approximately 4 hours as compared with fasted conditions.
Distribution
Trametinib is 97.4% bound to human plasma proteins. The apparent volume of distribution (Vc/F) is 214 L.
Elimination
The estimated elimination half-life is 3.9 to 4.8 days. The apparent clearance is 4.9 L/h.
Metabolism
Trametinib is metabolized predominantly via deacetylation alone or with mono- oxygenation or in combination with glucuronidation biotransformation pathways in vitro. Deacetylation is mediated by carboxylesterases (i.e., carboxylesterase 1b/c and 2) and may also be mediated by other hydrolytic enzymes.
Following a single dose of [14C]-trametinib, approximately 50% of circulating radioactivity is represented as the parent compound; however, ≥ 75% of drug- related material in plasma is the parent compound based on metabolite profiling after repeat dosing of trametinib.
Excretion
Following oral administration of [14C]-trametinib, greater than 80% of excreted radioactivity was recovered in the feces while less than 20% of excreted radioactivity was recovered in the urine with less than 0.1% of the excreted dose as parent.
Specific Populations
Age (18 to 93 years), sex, body weight (36 to 170 kg), and renal impairment (eGFR 15 to 89 mL/min/1.73 m2) have no clinically significant effect on the exposure of trametinib. There are insufficient data to evaluate potential differences in the exposure of trametinib by race or ethnicity.
Pediatric Patients: The pharmacokinetics of trametinib in glioma and other solid tumors were evaluated in 244 patients aged 1 to < 18 years following a single dose or multiple doses. Pharmacokinetic parameters in patients aged 1 to < 18 years are within range of values previously observed in adults given the same dose based on weight. Weight (6 to 156 kg) was found to have a statistically significant effect on trametinib oral clearance in this population.
Patients with Hepatic Impairment: Hepatic impairment (defined by bilirubin and AST levels) had no significant effect in trametinib exposure or apparent drug clearance compared with patients with normal hepatic function.
Drug Interaction Studies
Effect of Dabrafenib on Trametinib: Coadministration of MEKINIST tablets 2 mg daily with dabrafenib resulted in no change in AUC of trametinib.
Effect of Trametinib on CYP Substrates: Coadministration of MEKINIST tablets 2 mg once daily with a sensitive CYP3A4 substrate had no clinically relevant effect on the AUC and Cmax of the sensitive CYP3A4 substrate.
Based on in vitro studies, trametinib is an inhibitor of CYP2C8, but is not an inhibitor of CYP1A2, CYP2A6, CYP2B6, CYP2C9, CYP2C19, or CYP2D6 at a clinically relevant systemic concentration.
Effect of Transporters on Trametinib: Trametinib is a substrate of P-glycoprotein (P-gp) and BSEP. Inhibition of P-gp is unlikely to result in a clinically important increase in trametinib concentrations as trametinib exhibits high passive permeability and bioavailability. Trametinib is not a substrate of BCRP, OATP1B1, OATP1B3, OATP2B1, OCT1, MRP2, or MATE1 in vitro.
Effect of Trametinib on Transporters: Based on in vitro studies, trametinib is not an inhibitor of P-gp, BCRP, OATP1B1, OATP1B3, OAT1, OAT3, OCT2, BSEP, MRP2, or MATE1 at a clinically relevant systemic concentration.
NONCLINICAL TOXICOLOGY SECTION
13** NONCLINICAL TOXICOLOGY**
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies with trametinib have not been conducted. Trametinib was not genotoxic in studies evaluating reverse mutations in bacteria, chromosomal aberrations in mammalian cells, and micronuclei in the bone marrow of rats.
Trametinib may impair fertility in humans. In female rats given trametinib for up to 13 weeks, increased follicular cysts and decreased corpora lutea were observed at doses ≥ 0.016 mg/kg/day (approximately 0.3 times the human exposure at the recommended adult dose based on AUC). In rat and dog toxicity studies up to 13 weeks in duration, there were no treatment effects observed on male reproductive tissues [see Use in Specific Populations (8.3)].
INSTRUCTIONS FOR USE SECTION
|
This Instructions for Use has been approved by the U.S. Food and Drug Administration. |
Revised: March 2024 |
|
INSTRUCTIONS FOR USE | |
|
This “Instructions for Use” contains information on how to give MEKINIST oral solution. | |
|
Important information you need to know before giving MEKINIST oral solution | |
|
*MEKINIST oral solution should only be given by a caregiver.
| |
|
The MEKINIST pack should contain: | |
|
| |
|
Reusable oral syringe parts: | |
|
| |
|
Storage of MEKINIST oral solution | |
|
|
|
Storage of oral syringe | |
|
|
|
Section A. Measuring and giving a dose of MEKINIST oral solution | |
To give a dose of MEKINIST oral solution, you will need:
Call your healthcare provider or pharmacist if you do not have one or more of these items.
|
|
|
Step 1. Wash and dry your hands before measuring and giving a dose of MEKINIST oral solution. | |
|
Step 2. Place your supplies on a clean, flat work surface. | |
|
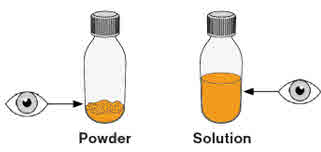
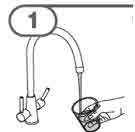
Step 3. Check if you have powder or solution in the amber bottle.
|
|
|
Step 4. Check the expiration date of the MEKINIST oral solution that is handwritten or printed on the amber bottle label. *Do not give MEKINIST oral solution if the expiration date has passed or there is no date on the amber bottle label. *Note: If you are unsure of the expiration date, contact your healthcare provider or pharmacist. |
|
|
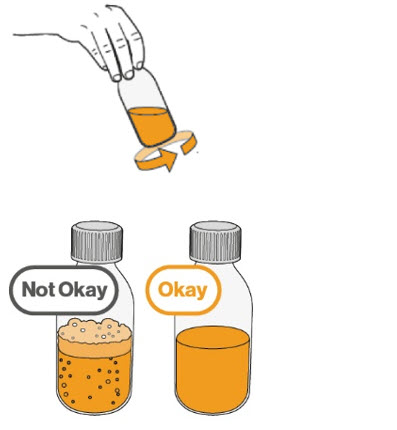
Step 5. Gently swirl the amber bottle for 30 seconds to mix the MEKINIST oral solution.
|
|
|
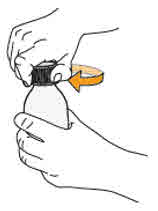
Step 6. When the foam has disappeared inside the amber bottle, remove the child-resistant cap by pushing down on the cap and turning it in the direction of the arrow (counter-clockwise), as shown. Then place the amber bottle back on your flat work surface. |
|
|
Step 7. Check if there is a bottle adapter already inserted in the bottle
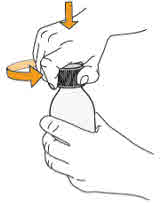
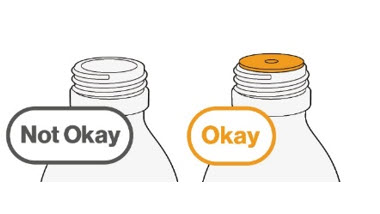
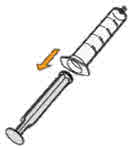
neck.Do not remove the bottle adapter. If not inserted, separate the
bottle adapter from the oral syringe and insert it. |
|
|
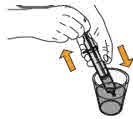
Step 8. Next, pick up the oral syringe. |
|
|
Step 9. Use one hand to hold the amber bottle containing the prepared MEKINIST oral solution steady. With your other hand, insert the tip of the oral syringe into the opening of the bottle adapter. Make sure the oral syringe is securely attached. Important: Due to air pressure, the plunger may move by itself when you measure your dose during Step 10. Hold the end of the plunger to prevent it from moving. |
|
|
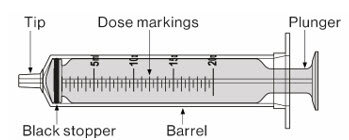
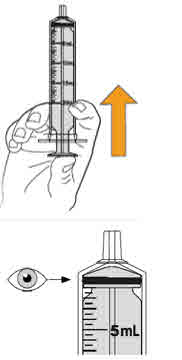
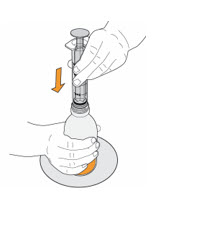
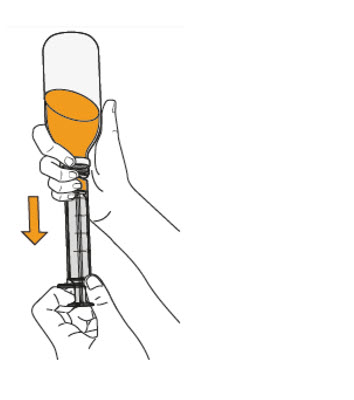
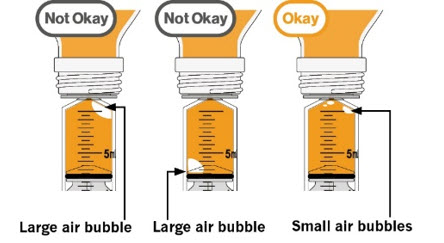
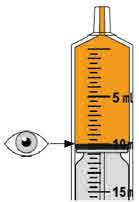
Step 10. Carefully turn the amber bottle upside down and pull down on the plunger to draw the MEKINIST oral solution into the oral syringe. To measure your dose, keep the tip of the oral syringe facing up. Pull down on the plunger until the top of the black stopper lines up with your prescribed dose in mLs on the oral syringe barrel. If large air bubbles appear in the oral syringe, push the MEKINIST oral solution back into the amber bottle and then pull down on the plunger again to draw up your dose. |
|
|
Keep doing this until there are no large air bubbles present. Small air bubbles are okay. |
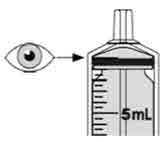
|
|
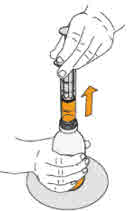
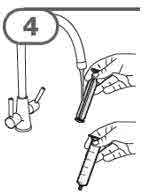
Step 11. Continue to hold the plunger in place, and carefully turn the
amber bottle upright. Put the amber bottle onto your flat work surface again. |
|
|
Check again to be sure that the top of the black stopper is at your prescribed
dose. If not, repeat Steps 9, 10 and 11 again. |
|
|
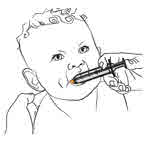
Step 12. Important: If giving a dose of MEKINIST oral solution to a child,
make sure they are sitting upright. Place the tip of the oral syringe inside
the mouth. The tip should touch the inside of either cheek. |
|
|
Step 13. Check to make sure that there is no MEKINIST oral solution left
in the oral syringe. |
|
|
Step 14. When finished, close the amber bottle. |
|
|
Step 15. Clean the reusable oral syringe. See the instructions in Section C “Cleaning the reusable oral syringe”. |
|
|
Section B. Giving a dose of MEKINIST oral solution through a feeding tube | |
|
This section is for useonly if you are going to give a dose of MEKINIST
oral solution through afeeding tube.
| |
|
Step 1. Flush the feeding tube according to the manufacturer’s instructions right away before giving a dose of MEKINIST oral solution. | |
|
Step 2. Follow Steps 1 through 11 in Section A, then move to Step 3 in this section. | |
|
Step 3. Connect the 20 mL oral syringe containing MEKINIST oral solution to the feeding tube. You may need an ENFIT adapter to connect the oral syringe to the feeding tube. | |
|
Step 4. Apply steady pressure to the plunger to give the dose of MEKINIST oral solution through the feeding tube. | |
|
Step 5. Check to be sure that there is no MEKINIST oral solution left in
the oral syringe. | |
|
Step 6. Flush the feeding tube again according to the manufacturer’s instructions. | |
|
Step 7. Go to Section C for instructions on “Cleaning the reusable oral syringe”. | |
|
Section C. Cleaning the reusable oral syringe | |
|
Note: Clean the oral syringe separate from other kitchen items. | |
|
Step 1. Fill a glass with warm, soapy water. |
|
|
Step 2. Place the tip of the oral syringe into the glass of warm, soapy
water. |
|
|
Step 3. Remove the plunger from the barrel. |
|
|
Step 4. Rinse the glass, plunger, and barrel under warm tap water. |
|
|
Step 5. Leave the oral syringe plunger and barrel on a clean paper towel
to air dry. When your oral syringe is dry, store it with the amber bottle of
MEKINIST oral solution. |
|
|
How to throw away MEKINIST oral solution that is expired or no longer needed, or old oral syringes | |
| |
|
How to clean up any spilled MEKINIST oral solution | |
|
If you accidentally spill any MEKINIST oral solution, clean up the spill as
follows: | |
|
Distributed by: © Novartis |
T2024-27