Icosapent Ethyl
These highlights do not include all the information needed to use ICOSAPENT ETHYL CAPSULES safely and effectively. See full prescribing information for ICOSAPENT ETHYL CAPSULES. ICOSAPENT ETHYL capsules, for oral use Initial U.S. Approval: 2012
886aa8d8-b293-258f-b16d-be40418787a8
HUMAN PRESCRIPTION DRUG LABEL
Dec 14, 2023
Apotex Corp
DUNS: 845263701
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Icosapent Ethyl
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
Drug Labeling Information
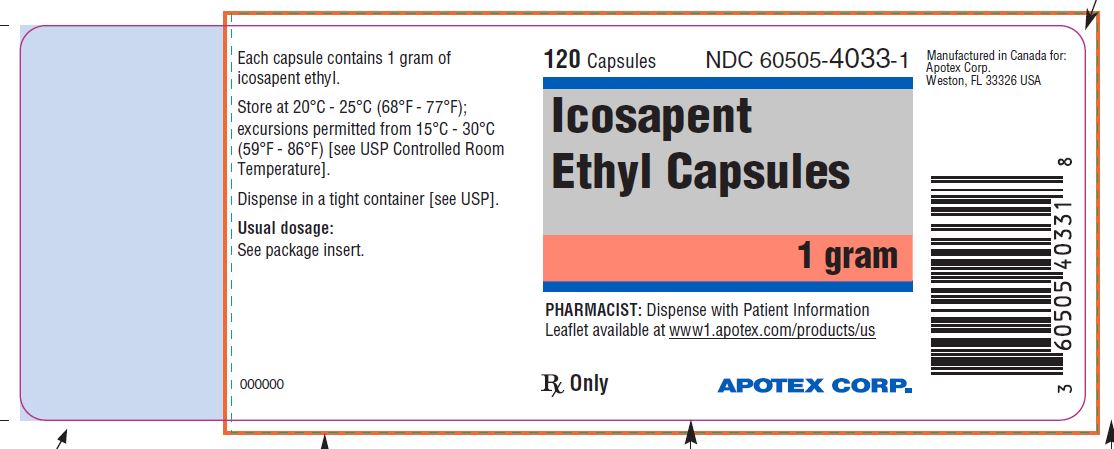
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 120 Capsule Bottle Label
NDC 60505-4033-1
Icosapent Ethyl Capsules
1 gram
120 capsules
Rx only
SPL PATIENT PACKAGE INSERT SECTION
PATIENT INFORMATION
Icosapent Ethyl Capsules
(eye koe’ sa pent eth’ il)
Patient Information Leaflet available at www.apotex.com/products/us/mg.asp
What are icosapent ethyl capsules?
Icosapent ethyl capsules are a prescription medicine used:
- along with a low-fat and low-cholesterol diet to lower high levels of triglycerides (fats) in adults.
It is not known if icosapent ethyl capsules change your risk of having inflammation of your pancreas (pancreatitis).
It is not known if icosapent ethyl capsules are safe and effective in children.
Do not take icosapent ethyl capsules if you are allergic to icosapent ethyl or any of the ingredients in icosapent ethyl capsules. See the end of this leaflet for a complete list of ingredients in icosapent ethyl capsules.
Before taking icosapent ethyl capsules, tell your doctor**** about all of your medical conditions, including****** if you:**
- have diabetes.
- have a low thyroid problem (hypothyroidism).
- have a liver problem.
- have a pancreas problem.
- are allergic to fish or shellfish. It is not known if people who are allergic to fish or shellfish are also allergic to icosapent ethyl capsules.
- are pregnant, or planning to become pregnant. It is not known if icosapent ethyl capsules will harm your unborn baby.
- are breastfeeding or plan to breastfeed. Icosapent ethyl can pass into your breastmilk, and may harm your baby. Talk to your doctor about the best way to feed your baby if you take icosapent ethyl capsules.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and dietary or herbal supplements.
Icosapent ethyl capsules can interact with certain other medicines that you are taking.
Especially tell your doctor if you take medicines that affect your blood clotting (anticoagulants or blood thinners).
How should I take icosapent ethyl capsules?
-
Take icosapent ethyl capsules exactly as your doctor tells you to take it.
-
Do not change your dose or stop taking icosapent ethyl capsules without talking to your doctor.
-
Do not take more capsules than what is prescribed by your doctor.
- If you are prescribed the 1 gram capsules, you should not take more than 4 capsules each day with food.
-
Take icosapent ethyl capsules whole. Do not break, crush, dissolve, or chew icosapent ethyl capsules before swallowing.
-
If you miss a dose of icosapent ethyl capsules, take it as soon as you remember. However, if you miss one day of icosapent ethyl capsules, do not double your dose when you take it.
-
Your doctor may start you on a diet that is low in saturated fat, cholesterol, carbohydrates, and low in added sugars before giving you icosapent ethyl capsules. Stay on this diet while taking icosapent ethyl capsules.
-
Your doctor may do blood tests to check your triglyceride and other lipid levels while you take icosapent ethyl capsules.
What are the possible side effects of icosapent ethyl capsules?
Icosapent ethyl capsules may cause serious side effects, including:
***Heart rhythm problems (atrial fibrillation and atrial flutter).**Heart rhythm problems which can be serious and cause hospitalization have happened in people who take icosapent ethyl capsules, especially in people who have heart (cardiovascular) disease or diabetes with a risk factor for heart (cardiovascular) disease, or who have had heart rhythm problems in the past. Tell your doctor if you get any symptoms of heart rhythm problems such as feeling as if your heart is beating fast and irregular, lightheadedness, dizziness, shortness of breath, chest discomfort, or you faint.
***Possible allergic reactions if you are allergic to fish or shellfish.**Stop taking icosapent ethyl capsules and tell your doctor right away or get emergency medical help if you have any signs or symptoms of an allergic reaction.
*Bleeding. Serious bleeding can happen in people who take icosapent ethyl capsules. Your risk of bleeding may increase if you are also taking a blood thinner medicine.
If you have liver problems and are taking icosapent ethyl capsules, your doctor should do blood tests during treatment.
The most common side effect of icosapent ethyl capsules include:
- Muscle and joint pain.
- Swelling of the hands, legs, or feet.
- Constipation
- Gout
- Heart rhythm problems (atrial fibrillation).
These are not all the possible side effects of icosapent ethyl capsules. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1- 800-FDA-1088.
How should I storeicosapent ethyl capsules****?
- Store icosapent ethyl capsules at 68°F to 77° F (20°C to 25°C).
- Safely throw away medicine that is out of date or no longer needed.
Keepicosapent ethyl capsules**** and all medicine out of the reach of children.
General information about the safe and effective use oficosapent ethyl capsules****.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use icosapent ethyl capsules for a condition for which it was not prescribed. Do not give icosapent ethyl capsules to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your pharmacist or healthcare provider for information about icosapent ethyl capsules that is written for health professionals.
For more information, go to www.apotex.com or call 1-800-706-5575.
What are the ingredients in icosapent ethyl capsules?
Active Ingredient: icosapent ethyl
Inactive Ingredients: gelatin, glycerine, purified water and tocopherol. The capsule imprinting ink contains ammonium hydroxide, propylene glycol, shellac, simethicone, and titanium dioxide.
This patient information has been approved by the U.S. Food and Drug Administration.
APOTEX INC.
ICOSAPENT ETHYL CAPSULES
1 gram
|
Manufactured in Canada for: |
|
Apotex Corp. |
|
Weston, FL 33326 USA |
Revised: May 2025
Rev: 7
DESCRIPTION SECTION
11 DESCRIPTION
Icosapent ethyl, a lipid-regulating agent, is supplied as a 1 gram oblong, natural colored transparent soft gelatin capsule for oral administration.
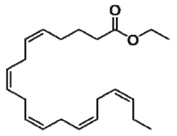
Each icosapent ethyl capsule contains 1 gram of icosapent ethyl. Icosapent ethyl is an ethyl ester of the omega-3 fatty acid eicosapentaenoic acid (EPA). The molecular formula of icosapent ethyl is C22H34O2 and the molecular weight is 330.5 g/mol. The chemical name for icosapent ethyl is ethyl (5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaenoate with the following chemical structure:
Icosapent ethyl capsules (1 gram capsules) also contain the following inactive ingredients: gelatin, glycerine, purified water and tocopherol. The capsule imprinting ink contains ammonium hydroxide, propylene glycol, shellac, simethicone, and titanium dioxide.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Icosapent ethyl capsules are supplied as 1 gram oblong, natural colored transparent soft gelatin capsules with the logo “A1000” printed in white ink.
Bottles of 120: NDC 60505-4033-1.
Store at 20°C to 25° C (68°F to 77°F); excursions permitted from 15°C to 30° C (59°F to 86°F) [see USP Controlled Room Temperature].
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling before starting icosapent ethyl capsules (Patient Information).
Inform patients that icosapent ethyl capsules may increase their risk for atrial fibrillation or atrial flutter [see Warnings and Precautions (5.1)].
Inform patients with known hypersensitivity to fish and/or shellfish about the potential for allergic reactions to icosapent ethyl capsules and advise them to discontinue icosapent ethyl capsules and seek medical attention if any reactions occur [see Warnings and Precautions (5.2)].
Inform patients that icosapent ethyl capsules may increase their risk for bleeding, especially if they are receiving other antithrombotic agents [see Warnings and Precautions (5.3)].
Advise patients to swallow icosapent ethyl capsules whole. Do not break open, crush, dissolve, or chew icosapent ethyl capsules [see Dosage and Administration (2.2)].
Instruct patients to take icosapent ethyl capsules as prescribed. If a dose is missed, patients should take it as soon as they remember. However, if they miss one day of icosapent ethyl capsules, they should not double the dose when they take it.
For more information about Icosapent Ethyl Capsules, go to www.apotex.com or call 1-800-706-5575.
Dispense with Patient Information Leaflet available at www.apotex.com/products/us/mg.asp
|
APOTEX INC |
|
ICOSAPENT ETHYL CAPSULES |
|
1 gram |
|
Manufactured in Canada for: |
|
Apotex Corp. |
|
Weston, FL 33326 USA |
Rev: 7
