Oxcarbazepine
These highlights do not include all the information needed to use OXCARBAZEPINE ORAL SUSPENSION safely and effectively. See full prescribing information for OXCARBAZEPINE ORAL SUSPENSION. OXCARBAZEPINE Oral Suspension, for oral administrationInitial U.S. Approval: 2000
1ccdb12e-3c52-4fb4-a2db-aeb8ca5757f7
HUMAN PRESCRIPTION DRUG LABEL
May 15, 2020
Sandoz Inc
DUNS: 005387188
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
oxcarbazepine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (14)
Drug Labeling Information
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Effect of Oxcarbazepine on Other Drugs
Phenytoin levels have been shown to increase with concomitant use of oxcarbazepine at doses greater than 1200 mg/day [see Clinical Pharmacology (12.3)]. Therefore, it is recommended that the plasma levels of phenytoin be monitored during the period of oxcarbazepine titration and dosage modification. A decrease in the dose of phenytoin may be required.
7.2 Effect of Other Drugs on Oxcarbazepine
Strong inducers of cytochrome P450 enzymes and/or inducers of UGT (e.g., rifampin, carbamazepine, phenytoin and phenobarbital) have been shown to decrease the plasma/serum levels of MHD, the active metabolite of oxcarbazepine (25% to 49%) [see Clinical Pharmacology (12.3)]. If oxcarbazepine and strong CYP3A4 inducers, or UGT inducers are administered concurrently, it is recommended that the plasma levels of MHD be monitored during the period of oxcarbazepine titration. Dose adjustment of oxcarbazepine may be required after initiation, dosage modification, or discontinuation of such inducers.
7.3 Hormonal Contraceptives
Concurrent use of oxcarbazepine with hormonal contraceptives may render these contraceptives less effective [see Use in Specific Populations (8.3) and Clinical Pharmacology (12.3)]. Studies with other oral or implant contraceptives have not been conducted.
•
Phenytoin: Increased phenytoin levels. Reduced dose of phenytoin may be required (7.1)
•
Carbamazepine, Phenytoin, and Phenobarbital: Decreased plasma levels of MHD (the active metabolite). Dose adjustments may be necessary (7.1)
•
Oral Contraceptive: Oxcarbazepine may decrease the effectiveness of hormonal contraceptives (7.3)
DRUG ABUSE AND DEPENDENCE SECTION
9 DRUG ABUSE AND DEPENDENCE
9.2 Abuse
The abuse potential of oxcarbazepine has not been evaluated in human studies.
9.3 Dependence
Intragastric injections of oxcarbazepine to 4 cynomolgus monkeys demonstrated no signs of physical dependence as measured by the desire to self-administer oxcarbazepine by lever pressing activity.
OVERDOSAGE SECTION
10 OVERDOSAGE
10.1 Human Overdose Experience
Isolated cases of overdose with oxcarbazepine have been reported. The maximum dose taken was approximately 48,000 mg. All patients recovered with symptomatic treatment. Nausea, vomiting, somnolence, aggression, agitation, hypotension, and tremor each occurred in more than one patient. Coma, confusional state, convulsion, dyscoordination, depressed level of consciousness, diplopia, dizziness, dyskinesia, dyspnea, QT prolongation, headache, miosis, nystagmus, overdose, decreased urine output, and blurred vision also occurred.
10.2 Treatment and Management
There is no specific antidote. Symptomatic and supportive treatment should be administered as appropriate. Removal of the drug by gastric lavage and/or inactivation by administering activated charcoal should be considered.
DESCRIPTION SECTION
11 DESCRIPTION
Oxcarbazepine is an AED available as a 300 mg/5 mL (60 mg/mL) oral suspension. Oxcarbazepine is 10,11-Dihydro-10-oxo-5H-dibenz[b,f]azepine-5-carboxamide, and its structural formula is:
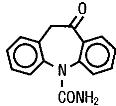
Oxcarbazepine is a white to faintly orange crystalline powder. It is slightly soluble in chloroform, dichloromethane, acetone, and methanol and practically insoluble in ethanol, ether and water. Its molecular weight is 252.27 g/mol.
Oxcarbazepine oral suspension contains the following inactive ingredients: ascorbic acid; dispersible cellulose; ethanol; macrogol stearate; methyl parahydroxybenzoate; propylene glycol; propyl parahydroxybenzoate; purified water; sodium saccharin; sorbic acid; sorbitol; yellow-plum-lemon aroma.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In 2-year carcinogenicity studies, oxcarbazepine was administered in the diet at doses of up to 100 mg/kg/day to mice and by gavage at doses of up to 250 mg/kg/day to rats, and the pharmacologically active 10-hydroxy metabolite (MHD) was administered orally at doses of up to 600 mg/kg/day to rats. In mice, a dose-related increase in the incidence of hepatocellular adenomas was observed at oxcarbazepine doses ≥ 70 mg/kg/day, which is less than the maximum recommended human dose (MRHD) on a mg/m2 basis. In rats, the incidence of hepatocellular carcinomas was increased in females treated with oxcarbazepine at doses ≥ 25 mg/kg/day (less than the MRHD on a mg/m2 basis), and incidences of hepatocellular adenomas and/or carcinomas were increased in males and females treated with MHD at doses of 600 mg/kg/day (2.4 times the MRHD on a mg/m2 basis) and ≥ 250 mg/kg/day (equivalent to the MRHD on a mg/m2 basis), respectively. There was an increase in the incidence of benign testicular interstitial cell tumors in rats at 250 mg oxcarbazepine/kg/day and at ≥ 250 mg MHD/kg/day, and an increase in the incidence of granular cell tumors in the cervix and vagina in rats at 600 mg MHD/kg/day.
Mutagenesis
Oxcarbazepine increased mutation frequencies in the in vitro Ames test in the absence of metabolic activation. Both oxcarbazepine and MHD produced increases in chromosomal aberrations and polyploidy in the Chinese hamster ovary assay in vitro in the absence of metabolic activation. MHD was negative in the Ames test, and no mutagenic or clastogenic activity was found with either oxcarbazepine or MHD in V79 Chinese hamster cells in vitro. Oxcarbazepine and MHD were both negative for clastogenic or aneugenic effects (micronucleus formation) in an in vivo rat bone marrow assay.
Impairment of Fertility
In a study in which male and female rats were administered oxcarbazepine (0, 25, 75, and 150 mg/kg/day) orally prior to and during mating and continuing in females during gestation, no adverse effects on fertility or reproductive performance were observed. The highest dose tested is less than the MRHD on a mg/m2 basis. In a fertility study in which rats were administered MHD (0, 50, 150, or 450 mg/kg/day) orally prior to and during mating and early gestation, estrous cyclicity was disrupted and numbers of corpora lutea, implantations, and live embryos were reduced in females receiving the highest dose (approximately 2 times the MRHD on a mg/m2 basis).
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Adjunctive Therapy for Adults
Initiate oxcarbazepine with a dose of 600 mg/day, given twice a day. If clinically indicated, the dose may be increased by a maximum of 600 mg/day at approximately weekly intervals; the maximum recommended daily dose is 1200 mg/day. Daily doses above 1200 mg/day show somewhat greater effectiveness in controlled trials, but most patients were not able to tolerate the 2400 mg/day dose, primarily because of central nervous (CNS) effects.
Dosage adjustment is recommended with concomitant use of strong CYP3A4 enzyme inducers or UGT inducers, which include certain antiepileptic drugs (AEDs) [see Drug Interactions (7.1, 7.2)].
2.2 Conversion to Monotherapy for Adults
Patients receiving concomitant AEDs may be converted to monotherapy by initiating treatment with oxcarbazepine at 600 mg/day (given in a twice a day regimen) while simultaneously initiating the reduction of the dose of the concomitant AEDs. The concomitant AEDs should be completely withdrawn over 3 to 6 weeks, while the maximum dose of oxcarbazepine should be reached in about 2 to 4 weeks. Oxcarbazepine may be increased as clinically indicated by a maximum increment of 600 mg/day at approximately weekly intervals to achieve the maximum recommended daily dose of 2400 mg/day. A daily dose of 1200 mg/day has been shown in one study to be effective in patients in whom monotherapy has been initiated with oxcarbazepine. Patients should be observed closely during this transition phase.
2.3 Initiation of Monotherapy for Adults
Patients not currently being treated with AEDs may have monotherapy initiated with oxcarbazepine. In these patients, initiate oxcarbazepine at a dose of 600 mg/day (given a twice a day); the dose should be increased by 300 mg/day every third day to a dose of 1200 mg/day. Controlled trials in these patients examined the effectiveness of a 1200 mg/day dose; a dose of 2400 mg/day has been shown to be effective in patients converted from other AEDs to oxcarbazepine monotherapy (see above).
2.4 Adjunctive Therapy for Pediatric Patients (Aged 2-16 Years)
In pediatric patients aged 4–16 years, initiate oxcarbazepine at a daily dose of 8 to 10 mg/kg generally not to exceed 600 mg/day, given twice a day. The target maintenance dose of oxcarbazepine should be achieved over 2 weeks, and is dependent upon patient weight, according to the following chart:
20 to 29 kg–900 mg/day
29.1 to 39 kg–1200 mg/day
> 39 kg–1800 mg/day
In the clinical trial, in which the intention was to reach these target doses, the median daily dose was 31 mg/kg with a range of 6 to 51 mg/kg.
In pediatric patients aged 2 to < 4 years, initiate oxcarbazepine at a daily dose of 8 to 10 mg/kg generally not to exceed 600 mg/day, given twice a day. For patients less than 20 kg, a starting dose of 16 to 20 mg/kg may be considered [see Clinical Pharmacology (12.3)]. The maximum maintenance dose of oxcarbazepine should be achieved over 2 to 4 weeks and should not exceed 60 mg/kg/day in a twice a day regimen.
In the clinical trial in pediatric patients (2 to 4 years of age), in which the intention was to reach the target dose of 60 mg/kg/day, 50% of patients reached a final dose of at least 55 mg/kg/day.
Under adjunctive therapy (with and without enzyme-inducing AEDs), when normalized by body weight, apparent clearance (L/hr/kg) decreased when age increased such that children 2 to < 4 years of age may require up to twice the oxcarbazepine dose per body weight compared to adults; and children 4 to ≤ 12 years of age may require a 50% higher oxcarbazepine dose per body weight compared to adults.
Dosage adjustment is recommended with concomitant use of strong CYP3A4 enzyme inducers or UGT inducers, which include certain AEDs [see Drug Interactions (7.1, 7.2)].
2.5 Conversion to Monotherapy for Pediatric Patients (Aged 4-16 Years)
Patients receiving concomitant AEDs may be converted to monotherapy by initiating treatment with oxcarbazepine at approximately 8 to 10 mg/kg/day given twice a day, while simultaneously initiating the reduction of the dose of the concomitant AEDs. The concomitant AEDs can be completely withdrawn over 3 to 6 weeks, while oxcarbazepine may be increased as clinically indicated by a maximum increment of 10 mg/kg/day at approximately weekly intervals to achieve the recommended daily dose. Patients should be observed closely during this transition phase.
The recommended total daily dose of oxcarbazepine is shown in Table 1.
2.6 Initiation of Monotherapy for Pediatric Patients (Aged 4-16 Years)
Patients not currently being treated with AEDs may have monotherapy initiated with oxcarbazepine. In these patients, initiate oxcarbazepine at a dose of 8 to 10 mg/kg/day given twice a day. The dose should be increased by 5 mg/kg/day every third day to the recommended daily dose shown in the table below.
Table 1: Range of Maintenance Doses of Oxcarbazepine for Pediatrics by Weight During Monotherapy|
From |
To | |
|
Weight in kg |
Dose (mg/day) |
Dose (mg/day) |
|
600 |
900 |
|
900 |
1200 |
|
900 |
1200 |
|
900 |
1500 |
|
900 |
1500 |
|
1200 |
1500 |
|
1200 |
1800 |
|
1200 |
1800 |
|
1200 |
2100 |
|
1200 |
2100 |
|
1500 |
2100 |
2.7 Dosage Modification for Patients With Renal Impairment
In patients with impaired renal function (creatinine clearance < 30 mL/min), initiate oxcarbazepine at one-half the usual starting dose (300 mg/day, given twice a day), and increase slowly to achieve the desired clinical response [see Clinical Pharmacology (12.3)].
2.8 Administration Information
Oxcarbazepine can be taken with or without food [see Clinical Pharmacology (12.3)].
Before using oxcarbazepine oral suspension, shake the bottle well and prepare the dose immediately afterwards. The prescribed amount of oral suspension should be withdrawn from the bottle using the oral dosing syringe supplied. Oxcarbazepine oral suspension can be mixed in a small glass of water just prior to administration, or alternatively, may be swallowed directly from the syringe. After each use, close the bottle and rinse the syringe with warm water, and allow it to dry thoroughly.
Oxcarbazepine oral suspension and oxcarbazepine film‑coated tablets may be interchanged at equal doses.
Adults: initiate with a dose of 600 mg/day, given twice a day
•
Adjunctive Therapy: Maximum increment of 600 mg/day at approximately weekly intervals. The recommended daily dose is 1200 mg/day (2.1)
•
Conversion to Monotherapy: Withdrawal concomitant over 3 to 6 weeks; reach maximum dose of oxcarbazepine in 2 to 4 weeks with increments of 600 mg/day at weekly intervals to a recommended daily dose of 2400 mg/day (2.2)
•
Initiation of Monotherapy: Increments of 300 mg/day every third day to a dose of 1200 mg/day (2.3)
•
Initiate at one-half the usual starting dose and increase slowly in patients with a creatinine clearance < 30 mL/min (2.7)
Pediatrics: initiation with 8 to 10 mg/kg/day, given twice a day. For patients aged 2 to <4 years and under 20 kg, a starting dose of 16 to 20 mg/kg/day may be considered. Recommended daily dose is dependent upon patient weight.
•
Adjunctive Patients (Aged 2-16 Years): For patients aged 4 to 16 years, target maintenance dose should be achieved over 2 weeks (2.4). For patients aged 2 to <4 years, maximum maintenance dose should be achieved over 2 to 4 weeks and should not to exceed 60 mg/kg/day (2.4)
•
Conversion to Monotherapy for Patients (Aged 4-16 Years): Maximum increment of 10 mg/kg/day at weekly intervals, concomitant antiepileptic drugs (AEDs) can be completely withdrawn over 3 to 6 weeks (2.5)
•
Initiation of Monotherapy for Patients (Aged 4-16 Years): Increments of 5 mg/kg/day every third day (2.6)
