Denorex Severe Itch
Denorex Severe Itch
213ccbc4-2f52-58b6-e063-6294a90ae248
HUMAN OTC DRUG LABEL
Sep 3, 2024
Neoteric Cosmetics, Inc.
DUNS: 790615181
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Coal Tar
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (22)
Drug Labeling Information
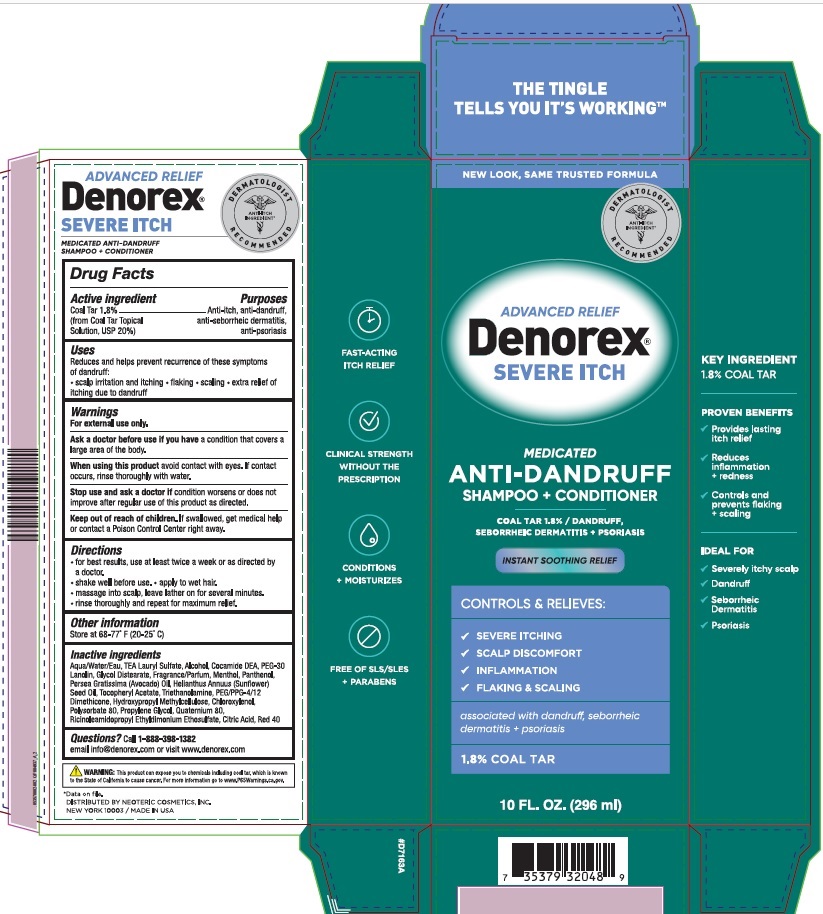
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
ADVANCED RELIEF
Denorex®
SEVERE ITCH
MEDICATED
ANTI-DANDRUFF
SHAMPOO + CONDITIONER
1.8 % Coal Tar / DANDRUFF,
SEBORRHEIC DERMATITIS + PSORIASIS
INSTANT SOOTHING RELIEF
CONTROLS & RELIEVES:
SEVERE ITCHING
SCALP DISCOMFORT
INFLAMMATION
FLAKING & SCALING
associated with dandruff, seborrheic dermatitis + psoriasis
1.8% COAL TAR
10 FL OZ (296 mL)
INDICATIONS & USAGE SECTION
Uses**:**
Reduces and helps prevent recurrence of these symptoms of dandruff:
- scalp irritation and itching • flaking • scaling • extra relief of itching due to dandruff
SPL UNCLASSIFIED SECTION
WARNING**:**
This product can expose you to chemicals including coal tar, which is known to the State of California to cause cancer. For more information go to www.P65Warnings.ca.gov.
OTC - ACTIVE INGREDIENT SECTION
Active Ingredients**:**
Coal Tar 1.8%
(from Coal Tar Topical Solution, USP 20%)
OTC - PURPOSE SECTION
Purpose**:**
Anti-itch, anti-dandruff, anti-seborrheic dermatitis, anti-psoriasis
WARNINGS SECTION
Warnings**:**
For external use only
Ask a doctor before use if you have
a condition covers a large area of the body.
When using this product
avoid contact with eyes. If contact occurs, rinse thoroughly with water
Stop use and ask a doctor
if condition worsens or does not improve after regular use of this product as directed.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
DOSAGE & ADMINISTRATION SECTION
Directions**:**
- for best results, use at least twice a week or as directed by a doctor
- shake well before use
- apply to wet hair
- massage into scalp, leave lather on for several minutes
- rinse thoroughly and repeat for maximum relief
INACTIVE INGREDIENT SECTION
Inactive ingredients
Aqua/Water/Eau, TEA Lauryl Sulfate, Alcohol, Cocamide DEA, PEG-30 Lanolin, Glycol Distearate, Fragrance/Parfum, Menthol, Panthenol, Persea Gratissima (Avocado) Oil, Helianthus Annuus (Sunflower) Seed Oil, Tocopheryl Acetate, Triethanolamine, PEG/PPG-4/12 Dimethicone, Hydroxypropyl Methylcellulose, Chloroxylenol, Polysorbate 80, Propylene Glycol, Quaternium 80, Ricinoleamidopropyl Ethyldimonium Ethosulfate, Citric Acid, Red 40
OTC - QUESTIONS SECTION
Questions?
Call1-888-398-1382
email info@denorex.com or visit www.denorex.com
