Econazole Nitrate
Econazole Nitrate Cream 1%
90c40d7f-94fa-440c-9815-30378f76ae79
HUMAN PRESCRIPTION DRUG LABEL
Aug 26, 2025
Sun Pharmaceutical Industries, Inc.
DUNS: 146974886
Taro Pharmaceuticals U.S.A., Inc.
DUNS: 145186370
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Econazole Nitrate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (18)
Drug Labeling Information
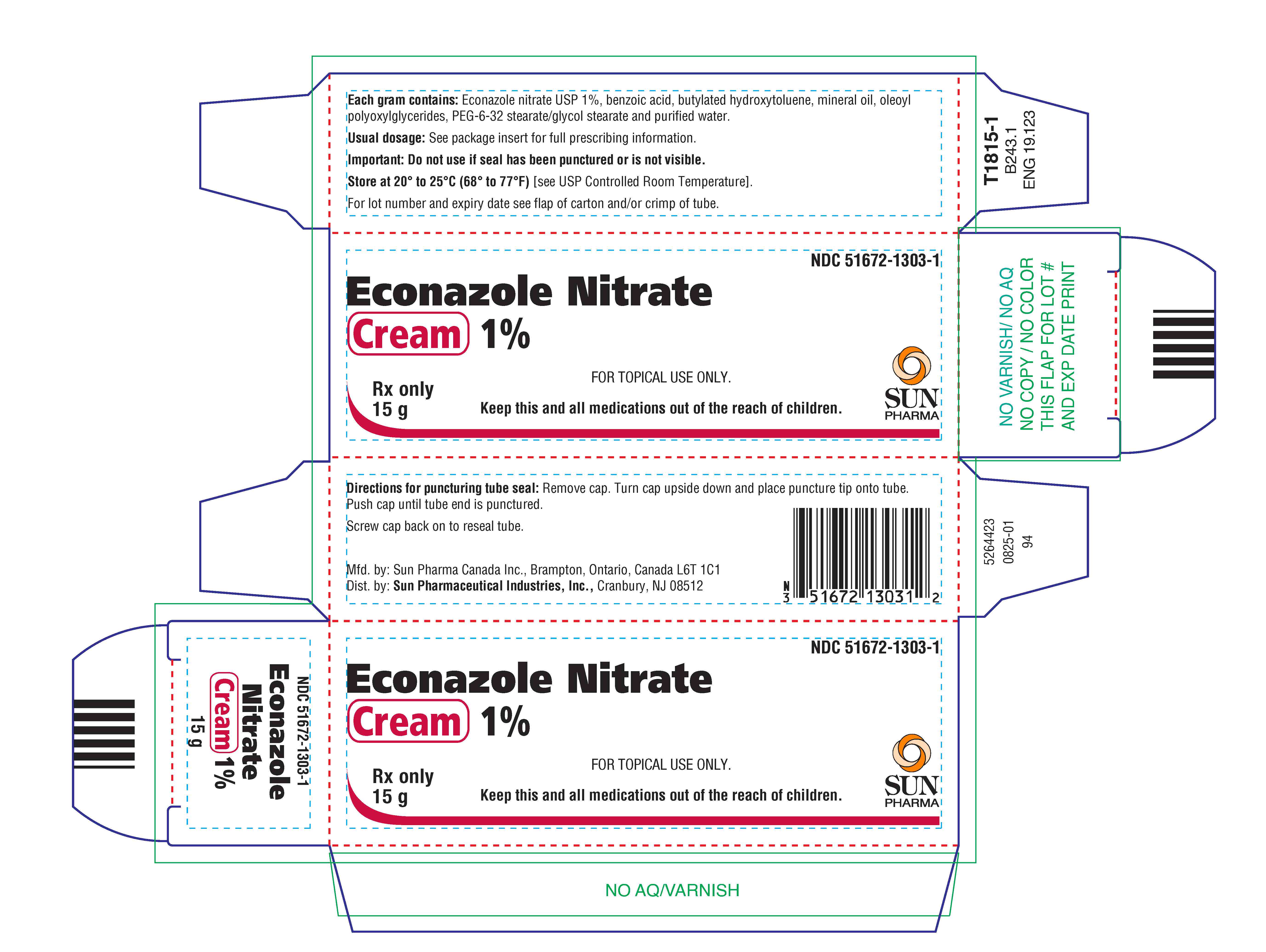
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 15 g Tube Carton
INDICATIONS & USAGE SECTION
INDICATIONS AND USAGE
Econazole nitrate cream is indicated for topical application in the treatment of tinea pedis, tinea cruris, and tinea corporis caused by Trichophyton rubrum, Trichophyton mentagrophytes, Trichophyton tonsurans, Microsporum canis, Microsporum audouini, Microsporum gypseum,and Epidermophyton floccosum,in the treatment of cutaneous candidiasis, and in the treatment of tinea versicolor.
HOW SUPPLIED SECTION
HOW SUPPLIED
Econazole Nitrate Cream, 1% is supplied in tubes of 15 g (NDC 51672-1303-1), 30 g (NDC 51672-1303-2) and 85 g (NDC 51672-1303-8).
Store at 20° to 25°C (68° to 77°F)[see USP Controlled Room Temperature].
CLINICAL PHARMACOLOGY SECTION
CLINICAL PHARMACOLOGY
After topical application to the skin of normal subjects, systemic absorption of econazole nitrate is extremely low. Although most of the applied drug remains on the skin surface, drug concentrations were found in the stratum corneum, which, by far, exceeded the minimum inhibitory concentration for dermatophytes. Inhibitory concentrations were achieved in the epidermis and as deep as the middle region of the dermis. Less than 1% of the applied dose was recovered in the urine and feces.
Microbiology
Econazole nitrate has been shown to be active against most strains of the following microorganisms, both in vitroand in clinical infections as described in theINDICATIONS AND USAGEsection.
|
Dermatophytes |
Yeasts |
|---|---|
|
Epidermophyton floccosum |
Candida albicans |
|
Microsporum audouini |
Malassezia furfur |
|
Microsporum canis | |
|
Microsporum gypseum | |
|
Trichophyton mentagrophytes | |
|
Trichophyton rubrum | |
|
Trichophyton tonsurans |
Econazole nitrate exhibits broad-spectrum antifungal activity against the following organisms in vitro,but the clinical significance of these data is unknown.
|
Dermatophytes |
Yeasts |
|---|---|
|
Trichophyton verrucosum |
Candida guillermondii |
|
Candida parapsilosis | |
|
Candida tropicalis |
SPL UNCLASSIFIED SECTION
To report SUSPECTED ADVERSE REACTIONS, contact Sun Pharmaceutical Industries, Inc., at 1-866-923-4914 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch for voluntary reporting of adverse reactions.
Mfd. by: Sun Pharma Canada Inc., Brampton, Ontario, Canada L6T 1C1
Dist. by:** Sun Pharmaceutical Industries, Inc.,** Cranbury, NJ 08512
Revised: August 2025 5264354-0825-01
DESCRIPTION SECTION
DESCRIPTION
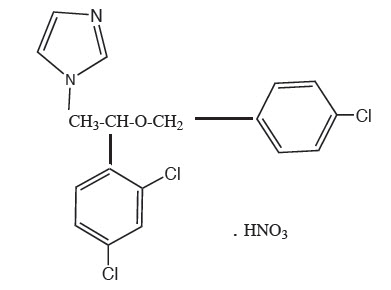
Econazole nitrate cream contains the antifungal agent, econazole nitrate USP 1%, in a water-miscible base consisting of benzoic acid, butylated hydroxytoluene, mineral oil, oleoyl polyoxylglycerides PEG-6-32 stearate/glycol stearate and purified water. The white to off-white soft cream is for topical use only.
Chemically, econazole nitrate is 1-[2-{(4-chloro-phenyl) methoxy}-2-(2,4-dichlorophenyl)ethyl]-1H-imidazole mononitrate. Its structure is as follows: