Nail Fungus Treatment
3b341440-9df2-bfb6-e063-6294a90a4c82
HUMAN OTC DRUG LABEL
Jul 31, 2025
TT Beauty Trading Co., Ltd.
DUNS: 661400516
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
GLYCERIN
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
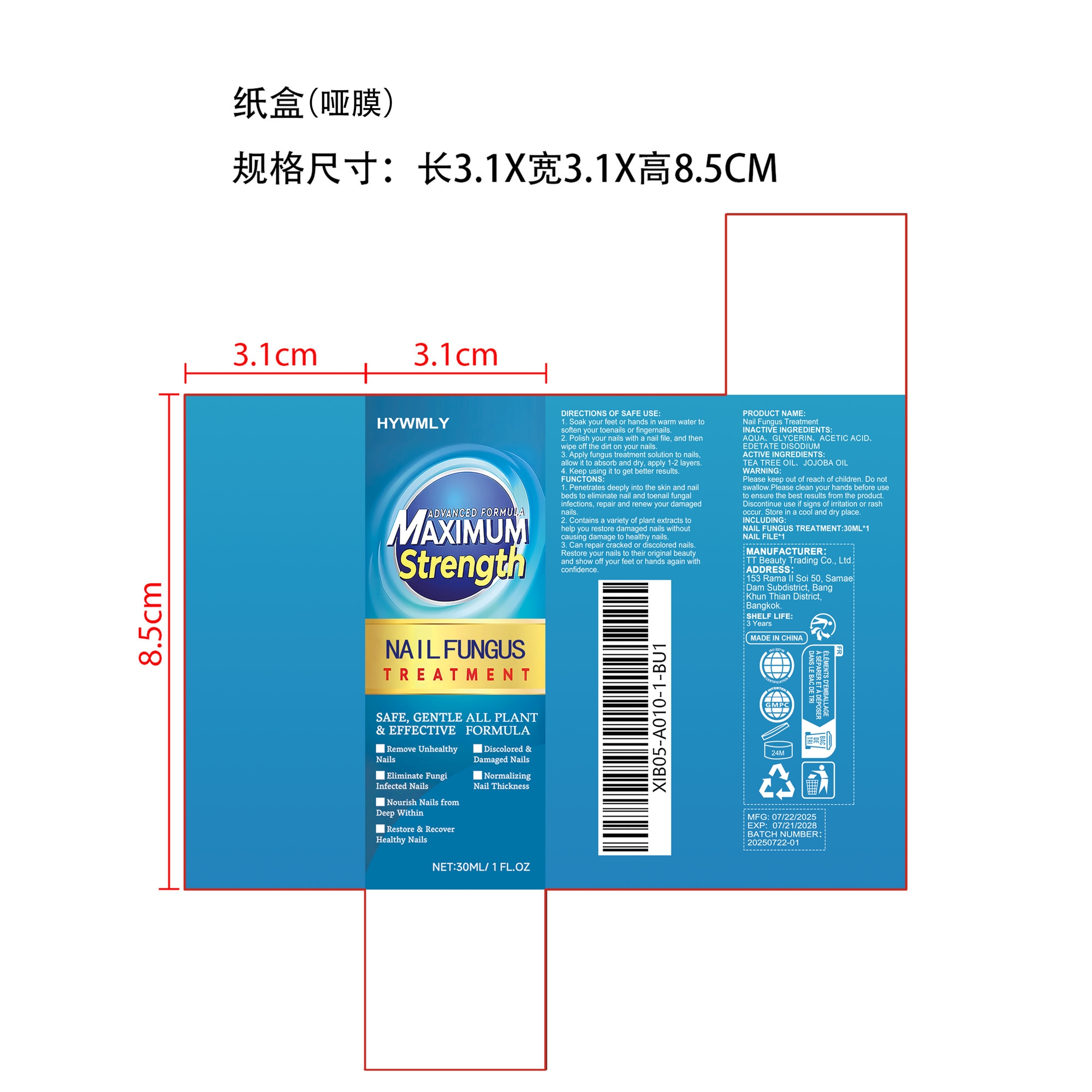
INDICATIONS & USAGE SECTION
Can repair cracked or discolored nails. Restore your nails to their original beauty and show off your feet or hands again with confidence.
DOSAGE & ADMINISTRATION SECTION
Apply fungus treatment solution to nails, allow it to absorb and dry, apply 1-2 layers.
STORAGE AND HANDLING SECTION
Store in a cool and dry place.
OTC - ACTIVE INGREDIENT SECTION
TEA TREE OIL、 JOJOBA OIL
OTC - WHEN USING SECTION
1. Soak your feet or hands in warm water to soften your toenails or
fingernails.
2. Polish your nails with a nail file, and then wipe off the dirt on your
nails.
3. Apply fungus treatment solution to nails, allow it to absorb and dry,
apply 1-2 layers.
4. Keep using it to get better results.
OTC - DO NOT USE SECTION
Discontinue use if signs of irritation or rash occur.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Please keep out of reach of children. Do not swallow.
INACTIVE INGREDIENT SECTION
AQUA、
GLYCERIN、
ACETIC ACID、
EDETATE DISODIUM
OTC - PURPOSE SECTION
1. Penetrates deeply into the skin and nail beds to eliminate nail and
toenail fungal infections, repair and renew your damaged nails.
2. Contains a variety of plant extracts to help you restore damaged nails
without causing damage to healthy nails.
3. Can repair cracked or discolored nails. Restore your nails to their
original beauty and show off your feet or hands again with confidence.
WARNINGS SECTION
Please keep out of reach of children. Do not swallow.Please clean your hands before use to ensure the best results from the product. Discontinue use if signs of irritation or rash occur. Store in a cool and dry place.
OTC - STOP USE SECTION
Discontinue use if signs of irritation or rash occur.
