FUROSCIX
These highlights do not include all the information needed to use FUROSCIX safely and effectively. See full prescribing information for FUROSCIX. Furoscix® (furosemide injection), for subcutaneous use Initial U.S. Approval: 1968
eac958dd-8d43-e44e-e053-2995a90a4d5e
HUMAN PRESCRIPTION DRUG LABEL
Aug 12, 2025
scPharmaceuticals Inc.
DUNS: 079386513
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
furosemide injection 80 mg/ 10 mL
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (1)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Combination Product Physician Sample Primary Carton
NDC 71767-100-01
PHYSICIAN SAMPLE ONLY
NOT FOR SALE
1 X 10 mL Single Dose Prefilled Cartridge
1 X Single Use On-Body Infusor
FUROSCIX ®
(furosemide injection)
80 mg/10 mL
(8 mg/mL)
FOR SUBCUTANEOUS USE ONLY
Single Dose
Rx Only

INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
FUROSCIX is indicated for the treatment of edema in adult patients with chronic heart failure or chronic kidney disease (CKD), including the nephrotic syndrome.
FUROSCIX is a loop diuretic indicated for the treatment of edema in adult patients with chronic heart failure or chronic kidney disease, including the nephrotic syndrome. ( 1)
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
- FUROSCIX is contraindicated in patients with anuria.
- FUROSCIX is contraindicated in patients with a history of hypersensitivity to furosemide, any component of the FUROSCIX formulation, or medical adhesives.
- Anuria ( 4)
- Hypersensitivity to furosemide, components of FUROSCIX formulation, or medical adhesives. ( 4)
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Fluid, Electrolyte, and Metabolic Abnormalities
Furosemide may cause fluid, electrolyte, and metabolic abnormalities such as hypovolemia, hypokalemia, azotemia, hyponatremia, hypochloremic alkalosis, hypomagnesemia, hypocalcemia, hyperglycemia, or hyperuricemia, particularly in patients receiving higher doses, patients with inadequate oral electrolyte intake, and in elderly patients. Excessive diuresis may cause dehydration and blood volume reduction with circulatory collapse and possibly vascular thrombosis and embolism, particularly in elderly patients. Serum electrolytes, CO 2, BUN, creatinine, glucose, and uric acid should be monitored frequently during furosemide therapy [see Use in Specific Populations ( 8.6)] .
5.2 Worsening Renal Function
Furosemide can cause dehydration and azotemia. If increasing azotemia and oliguria occur during treatment of severe progressive renal disease, discontinue furosemide [see Clinical Pharmacology ( 12.3)].
5.3 Ototoxicity
Cases of tinnitus and reversible or irreversible hearing impairment and deafness have been reported with furosemide. Reports usually indicate that furosemide ototoxicity is associated with rapid injection, severe renal impairment, the use of higher than recommended doses, hypoproteinemia or concomitant therapy with aminoglycoside antibiotics, ethacrynic acid, or other ototoxic drugs. If the physician elects to use high-dose parenteral therapy, controlled intravenous infusion is advisable (for adults, an infusion rate not exceeding 4 mg furosemide per minute has been used) [see Drug Interactions ( 7.1)].
5.4 Acute Urinary Retention
In patients with severe symptoms of urinary retention (because of bladder emptying disorders, prostatic hyperplasia, urethral narrowing), the administration of furosemide can cause acute urinary retention related to increased production and retention of urine. These patients require careful monitoring, especially during the initial stages of treatment.
5.5 Incomplete Dosing
The On-body Infusor should not be allowed to get wet from water or any other fluids (blood or drug product). Fluid contact with the circuit board can lead to device errors and premature termination of infusion.
The On-body Infusor is intended for use in a setting where the patient can limit their activity for the duration of administration. Certain patient movements may cause interruption of device adherence to skin and premature termination of infusion.
The On-body Infusor for FUROSCIX should only be used in patients who can detect and respond to alarms to ensure a complete dose is administered.
- Fluid, Electrolyte, and Metabolic Abnormalities: Monitor serum electrolytes, CO 2, BUN, creatinine, glucose, and uric acid. ( 5.1)
- Worsening Renal Function: Monitor for dehydration and azotemia. ( 5.2)
- Ototoxicity: Avoid higher than recommended doses. ( 5.3, 7.1)
- Acute Urinary Retention: Monitor patients with symptoms of urinary retention. ( 5.4)
- Incomplete Dosing: Fluid contact and certain patient movements during treatment may cause the On-body Infusor to prematurely terminate infusion. Ensure patients can detect and respond to alarms. ( 5.5)
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Effects of Furosemide on Other Drugs
|
Drug/Substance Class or Name |
Drug Interaction Effect |
Recommendations |
|
Aminoglycoside antibiotics |
Furosemide may increase the ototoxic potential of aminoglycoside antibiotics, especially in the presence of impaired renal function [see Warnings and Precautions ( 5.3)]. |
Avoid combination except in life-threatening situations. |
|
Ethacrynic acid |
Possibility of ototoxicity [see Warnings and Precautions ( 5.3)]. |
Avoid concomitant use with ethacrynic acid. |
|
Salicylates |
May experience salicylate toxicity at lower doses because of competitive renal excretory sites. |
Monitor for symptoms of salicylate toxicity. |
|
Cisplatin |
There is a risk of ototoxic effects if cisplatin and furosemide are given concomitantly [see Warnings and Precautions ( 5.3)]. | |
|
Cisplatin and nephrotoxic drugs |
Nephrotoxicity |
Administer furosemide at lower doses and with positive fluid balance when used to achieve forced diuresis during cisplatin treatment. Monitor renal function. |
|
Paralytic agents |
Furosemide has a tendency to antagonize the skeletal muscle relaxing effect of tubocurarine and may potentiate the action of succinylcholine. |
Monitor for skeletal muscle effect. |
|
Lithium |
Furosemide reduces lithium’s renal clearance and add a high-risk of lithium toxicity. |
Avoid concomitant use with lithium. |
|
Angiotensin converting enzyme inhibitors or angiotensin II receptor blockers |
May lead to severe hypotension and deterioration in renal function, including renal failure. |
Monitor for changes in blood pressure and renal function and interrupt or reduce the dosage of furosemide, angiotensin converting enzyme inhibitors, or angiotensin receptor blockers if needed. |
|
Antihypertensive drugs |
Furosemide may add to or potentiate the therapeutic effect of other antihypertensive drugs. |
Monitor for changes in blood pressure and adjust the dose of other antihypertensive drugs if needed. |
|
Adrenergic blocking drugs or peripheral adrenergic blocking drugs |
Potentiation occurs. |
Monitor for changes in blood pressure and adjust the dose of adrenergic blocking drugs if needed. |
|
Norepinephrine |
Furosemide may decrease arterial responsiveness (vasoconstricting effect) to norepinephrine. |
Monitor blood pressure (or mean arterial pressure). |
|
Chloral hydrate |
In isolated cases, intravenous administration of furosemide within 24 hours of taking chloral hydrate may lead to flushing, sweating attacks, restlessness, nausea, increase in blood pressure, and tachycardia. |
Concomitant use with chloral hydrate is not recommended. |
|
Methotrexate and other drugs undergoing renal tubular secretion |
Furosemide may decrease renal elimination of other drugs that undergo tubular secretion. High-dose treatment of furosemide may result in elevated serum levels of these drugs and may potentiate their toxicity. |
Monitor serum levels of drugs undergoing renal tubular secretion and adjust the dose if needed. |
|
Cephalosporin |
Furosemide can increase the risk of cephalosporin-induced nephrotoxicity even in the setting of minor or transient renal impairment. |
Monitor for changes in renal function. |
|
Cyclosporine |
Increased risk of gouty arthritis secondary to furosemide-induced hyperuricemia and cyclosporine impairment of renal urate excretion. |
Monitor serum urate levels. |
|
Thyroid hormones |
High-doses (> 80 mg) of furosemide may inhibit the binding of thyroid hormones to carrier proteins and result in transient increase in free thyroid hormones, followed by an overall decrease in total thyroid hormone levels. |
Monitor the total thyroid hormone levels. |
7.2 Effect of Other Drugs on Furosemide
|
Drug/Substance Class or Name |
Drug Interaction Effect |
Recommendations |
|
Phenytoin |
Phenytoin interferes directly with renal action of furosemide. |
Monitor diuretic effects of furosemide and adjust the dose of furosemide if needed. |
|
Methotrexate and other drugs undergoing renal tubular secretion |
May reduce the effect of furosemide. High-dose treatment of methotrexate and these other drugs may result in elevated serum levels of furosemide and may potentiate the toxicity of furosemide. |
Monitor for enhanced toxicity of furosemide. |
|
Indomethacin |
Coadministration of indomethacin may reduce the natriuretic and antihypertensive effects of furosemide in some patients by inhibiting prostaglandin synthesis. Indomethacin may also affect plasma renin levels, aldosterone excretion, and renin profile evaluation. |
Patients receiving both indomethacin and furosemide should be observed closely to determine if the desired diuretic and/or antihypertensive effect of furosemide is achieved. |
- Aminoglycoside antibiotics: Increased potential ototoxicity of the antibiotics. Avoid combination. ( 7.1)
- Ethacrynic acid: Risk of ototoxicity. Avoid combination ( 7.1)
- Salicylates: Risk of salicylate toxicity. ( 7.1)
- Cisplatin and nephrotoxic drugs: Risk of ototoxicity and nephrotoxicity. ( 7.1)
- Lithium: Risk of lithium toxicity. ( 7.1)
- Renin-angiotensin inhibitors: Increased risk of hypotension and renal failure. ( 7.1)
- Adrenergic blocking drugs: Risk of potentiation. ( 7.1)
- Drugs undergoing renal tubular secretion: Risk of toxicity potentiation. ( 7.1)
See17 for PATIENT COUNSELING INFORMATION.
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from published observational studies, case reports, and post marketing reports, from decades of use, have not demonstrated a drug- associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes with furosemide use during pregnancy. Untreated congestive heart failure and cirrhosis of the liver can lead to adverse outcomes for the mother and the fetus (see Clinical Considerations).
In animal reproduction studies, furosemide has been shown to cause unexplained maternal deaths and abortions in rabbits when administered orally during organogenesis at 4 times a human i.v. dose of 80 mg based on body surface area (BSA) and oral bioavailability corrections, presumably secondary to volume depletion (see Data).
The estimated background risk for major birth defects and miscarriage for the indicated populations is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in the clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Disease-associated Maternal and/or Embryo/fetal Risk
Pregnant women with congestive heart failure are at increased risk for pre- term birth. Stroke volume and heart rate increase during pregnancy, increasing cardiac output, especially during the first trimester. Clinical classification of heart disease may worsen with pregnancy and lead to maternal death and/or stillbirth. Closely monitor pregnant patients for destabilization of their heart failure.
Pregnant women with symptomatic cirrhosis generally have poor outcomes including hepatic failure, variceal hemorrhage, pre-term delivery, fetal growth restriction and maternal death. Outcomes are worse with coexisting esophageal varices. Carefully monitor pregnant women with cirrhosis of the liver.
Data
Animal Data
The effects of furosemide on embryonic and fetal development and on pregnant dams were studied in mice, rats, and rabbits.
Furosemide caused unexplained maternal deaths and abortions in the rabbit at the lowest dose of 25 mg/kg (approximately 4 times the human i.v. dose of 80 mg based on BSA and oral bioavailability corrections). In another study, a dose of 50 mg/kg (approximately 7 times a human i.v. dose of 80 mg based on BSA and oral bioavailability corrections) also caused maternal deaths and abortions when administered to rabbits between Days 12 and 17 of gestation. In a third study, none of the pregnant rabbits survived an oral dose of 100 mg/kg. Data from the above studies indicate fetal lethality that can precede maternal deaths.
The results of the mouse study and one of the three rabbit studies also showed an increased incidence and severity of hydronephrosis (distention of the renal pelvis and, in some cases, of the ureters) in fetuses of treated dams as compared with the incidence of fetuses from the control group.
8.2 Lactation
Risk Summary
The presence of furosemide has been reported in human breast milk. There are no data on the effects on the breastfed infant or the effects on milk production. Doses of furosemide associated with clinically significant diuresis may impair milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for furosemide and any potential adverse effects on the breastfed infant from furosemide or from the underlying maternal condition.
8.4 Pediatric Use
Safety and efficacy for pediatric use have not been established [see Indications and Usage (1)].
8.5 Geriatric Use
Controlled clinical studies did not include sufficient numbers of subjects to determine whether subjects aged 65 and over respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for the elderly patients should be cautious, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
FUROSCIX is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function [see Clinical Pharmacology ( 12.3)].
8.6 Renal Impairment
Significantly reduced renal function can affect the response to furosemide. The On-body Infusor will deliver only an 80 mg dose of furosemide, and patients with significantly reduced renal function may require additional diuretic therapy.
8.7 Hepatic Impairment
In patients with hepatic cirrhosis and ascites, sudden alterations of fluid and electrolyte balance may precipitate hepatic encephalopathy and coma. Treat such patients in a setting where clinical status and electrolyte balance can be carefully monitored.
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
The single-use, On-body Infusor with prefilled cartridge is pre-programmed to deliver 30 mg of furosemide over the first hour followed by 12.5 mg per hour for the subsequent 4 hours [see Use in Specific Populations ( 8.6) and see Clinical Pharmacology ( 12)].
FUROSCIX is not for chronic use and should be replaced with oral diuretics as soon as practical.
2.2 Important Administration Instructions
FUROSCIX is intended for use in a setting where the patient can limit their activity for the duration of administration. [see Warnings and Precautions ( 5.5)]
FUROSCIX is not compatible with use in an MRI setting.
Inspect FUROSCIX prefilled cartridge prior to administration. FUROSCIX is a clear to slightly yellow solution. Do not use FUROSCIX if solution is discolored or cloudy [see Description ( 11)] .
Refer to the Instructions for Use for additional information.
Load the prefilled cartridge of furosemide into the On-body Infusor and close the cartridge holder.
Peel away the adhesive liner on the On-body Infusor and apply onto a clean, dry area of the abdomen between the top of the beltline and the bottom of the ribcage that is not tender, bruised, red or indurated. The distance from the top of the beltline to the bottom of the ribcage should be at least 2 ½ inches.
Start the injection by firmly pressing and releasing the blue start button.
Do not remove until the injection is complete (signaled by the solid green status light, beeping sound, and the white plunger rod filling the cartridge window).
Rotate the site of each subcutaneous administration.
- The single-use, On-body Infusor is pre-programmed to deliver 30mg of furosemide over the first hour then 12.5 mg per hour for the subsequent 4 hours. ( 2.1)
- FUROSCIX is not for chronic use and should be replaced with oral diuretics as soon as practical. ( 2.1)
- See Full Prescribing Information for important administration instructions. ( 2.2)
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
Injection: 80 mg/10 mL (8 mg/mL) as a clear to slightly yellow solution in a single-dose prefilled cartridge for use only with co-packaged single-use, On- body Infusor.
Injection: 80 mg/10 mL (8 mg/mL) in a single-dose prefilled cartridge co- packaged with a single-use On-body Infusor. ( 3)
DESCRIPTION SECTION
11 DESCRIPTION
FUROSCIX (furosemide injection 80 mg/10 mL) is a loop diuretic which is an anthranilic acid derivative.
Chemically, it is 4-chloro-N-furfuryl-5-sulfamoylanthranilic acid.
Furosemide is a white to slightly yellow crystalline powder. It is sparingly soluble in alcohol, freely soluble in dilute alkali solutions and insoluble in dilute acids. The structural formula is as follows:
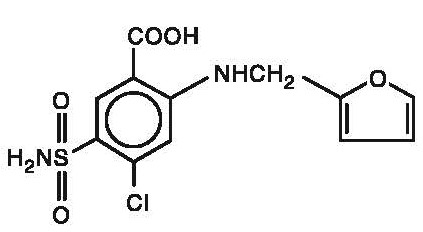
Molecular Formula: C 12H 11ClN 2O 5S
Molecular Weight: 330.75 g/mol
FUROSCIX is a single-dose prefilled cartridge co-packaged with a single-use, On-body Infusor. The single-dose prefilled cartridge contains 80 mg per 10 mL sterile, clear to slightly yellow, and non-pyrogenic furosemide solution. The pH of FUROSCIX, 7.4, differs from that of Furosemide Injection, USP.
Each 1 mL of FUROSCIX contains the following inactive ingredients: hydrochloric acid for pH adjustment if needed, sodium chloride (5.84 mg), sodium hydroxide for pH adjustment if needed, tris HCl (7.88 mg), and water for injection (q.s.).
FUROSCIX is administered via a wearable, single-use, electromechanical (battery powered, micro-processor controlled), On-body delivery system that is pre-programmed to deliver 80 mg of FUROSCIX over 5-hours using a bi-phasic delivery profile.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Furosemide primarily inhibits the reabsorption of sodium and chloride in the proximal and distal tubules and in the loop of Henle. The high degree of diuresis is largely due to the unique site of action. The action on the distal tubule is independent of any inhibitory effect on carbonic anhydrase and aldosterone.
12.2 Pharmacodynamics
In patients with NYHA Class II and Class III heart failure, subcutaneous administration of FUROSCIX (30 mg furosemide over the first hour followed by 12.5 mg per hour for the subsequent 4 hours, total 80mg furosemide) produced similar diuresis and natriuresis to intravenous administration (two 40 mg bolus doses separated by 120 minutes) at 8 and 24 hour post-dose. The duration of diuretic effect with FUROSCIX is up to 8 hours after initiation of dosing.
12.3 Pharmacokinetics
Absorption
In patients with NYHA Class II-III congestive heart failure, subcutaneous infusion of FUROSCIX (30 mg furosemide over the first hour followed by 12.5 mg per hour for the subsequent 4 hours, 80 mg furosemide total), the bioavailability was 99.6% (90% CI: 94.8, 104.8) with a median T max of 4 hours relative to 80 mg intravenous furosemide (two 40-mg bolus doses separated by 120 minutes). The pharmacokinetic parameters of FUROSCIX are presented in Table 1 below:
Table 1: Pharmacokinetic Data of FUROSCIX Following Subcutaneous Infusion (n = 15)
|
Dose |
C****max (ng/mL) |
AUCt (ng×hr/mL) |
T1/2 (hr) |
AUC**∞** (ng×hr/mL) |
|
FUROSCIX: 30 mg subcutaneously infused over the first hour followed by 12.5 mg per hour for the subsequent 4 hours (total dose: 80 mg furosemide) |
2040 ± 449 |
13000 ± 4000 |
3.2 ± 0.9 |
13100 ± 4010 |
|
Furosemide administered as 2 x 40 mg bolus doses intravenously, separated by 120 minutes (total dose: 80 mg furosemide) |
8580 ± 2540 |
13000 ± 4050 |
2.6 ± 0.3 |
13200 ± 4170 |
The terminal half-life of furosemide is approximately 2 hours.
The impact of subcutaneous edema at the administration site of FUROSCIX on drug absorption is unknown.
Distribution
Furosemide is extensively bound to plasma proteins, mainly to albumin. Plasma concentrations ranging from 1 mcg per mL to 400 mcg per mL are 91% to 99% bound in healthy individuals. The unbound fraction averages 2.3% to 4.1% at therapeutic concentrations.
Furosemide binding to albumin may be reduced in elderly patients.
Metabolism
Furosemide glucuronide is the only or at least the major biotransformation product of furosemide in man.
Elimination
Significantly more furosemide is excreted in urine following the intravenous injection than after the tablet or oral solution.
Furosemide is predominantly excreted unchanged in the urine.
The renal clearance of furosemide after intravenous administration in older healthy male subjects (60 to 70 years of age) is significantly less than in younger healthy male subjects (20 to 35 years of age).
INSTRUCTIONS FOR USE SECTION
INSTRUCTIONS FOR USE
Model/REF # FUR-80W5
Instructions For Use
On-Body Infusor for
FUROSCIX® [fue roe’ six]
(furosemide injection) for subcutaneous use
Single-Use On-Body Infusor and
80 mg/10 mL (8 mg/mL) Prefilled Cartridge
This Instructions for Use contains information about how to prepare and use FUROSCIX On-Body Infusor.
Before Starting
- Read this Instructions for Use before using the FUROSCIX On-Body Infusor for the first time.
- You or a caregiver should receive training before using the Furoscix On-Body Infusor.
- Always wash your hands before starting.
- The FUROSCIX On-Body Infusor will deliver a fixed dose of 80 mg of furosemide over about 5 hours.
- The FUROSCIX On-Body Infusor delivers the medicine just under the skin (subcutaneously).
- When the FUROSCIX On-Body Infusor is started, a small needle sticks into the skin to deliver the medicine. The on-body Infusor signals when all the medicine has been delivered. When the on-body Infusor is removed from the skin, a needle cover will extend over the needle to protect from an accidental needle stick.
Parts of the FUROSCIX On-Body Infusor and Prefilled Cartridge
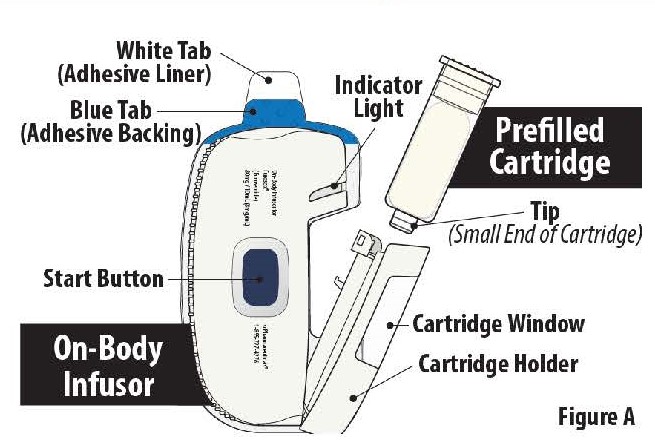
Important Information
- The on-body Infusor and prefilled cartridge are not made with natural rubber latex.
- The infusion will last about 5 hours. During this time, you should limit your activity so that your bending movements are limited. Wearing the Infusor while riding in a car or flying in an airplane is not recommended.
- You should notice an increase in urine production in about an hour after the infusion is started and may need to make frequent bathroom visits. Be sure you have access to a bathroom for up to 8 hours after starting the infusion. If you do not notice the need to go to the bathroom, call your healthcare provider.
- The on-body Infusor can only be used with the prefilled FUROSCIX cartridge supplied in the kit. *Do not use any other cartridges or medicines inside the on-body Infusor. *Do notapply lotions, oils, or ointments to the adhesion area of the abdomen prior to applying the on-body Infusor to the abdomen. *Do not use the on-body Infusor if the packaging appears to be opened, or if the on-body Infusor has been dropped or appears to be broken or damaged. *Do notuse the on-body Infusor within 12 inches of mobile phones, tablets, computers or wireless accessories (for example: TV remote control, Bluetooth computer keyboard or mouse). *Do not use the on-body Infusor or prefilled cartridge after the expiration date on the carton. *Do notuse the prefilled cartridge if the liquid is discolored or cloudy. The liquid in the prefilled cartridge should be clear to slightly yellow in color. *Do not use the on-body Infusor in or around an MRI machine. *Do nottake the on-body Infusor off your skin while it is infusing, unless there is an emergency or a device error. Removing the on-body Infusor before the infusion is done will result in a partial dose. The on-body Infusor will stop infusing and it cannot be restarted.Do not apply a new on-body Infusor unless instructed to do so by your healthcare provider. *Do notreuse the on-body Infusor. The on-body Infusor is for single use only. *Do not let the on-body Infusor get wet from water or any other liquids.Do not shower, bathe, swim or do activities that may make you sweat while wearing the on-body Infusor. It contains parts that should not get wet.
Call your healthcare provider or scPharmaceuticals at 1-855-SCPHARMA (1-855-727-4276) if you have any questions.
Storage Information
- The FUROSCIX On-Body Infusor and prefilled cartridge should be stored at controlled room temperature between 68°F to 77°F (20°C to 25°C) (see Figure B). Do not refrigerate or freeze. *Keep the FUROSCIX prefilled cartridge in the original carton to protect from light or physical damage until you are ready to use. *Keep the FUROSCIX On-Body Infusor and prefilled cartridge out of the reach of children.
Prepare for the Infusion
STEP 1 Wash Hands, Check Expiration Date and Remove Supplies from Carton
1.1Wash your hands before using the on-body Infusor (Figure C).
1.2 Check the expiration date on the FUROSCIX On-Body Infusor carton (see Figure D).Do notuse if the expiration date has passed.
1.3Open the carton and peel away the white paper cover. Remove the plastic cover from the clear tray. Remove supplies from the carton and packaging (see Figure E).Do not use the on-body Infusor or prefilled cartridge if packaging appears to have been previously opened or damaged.
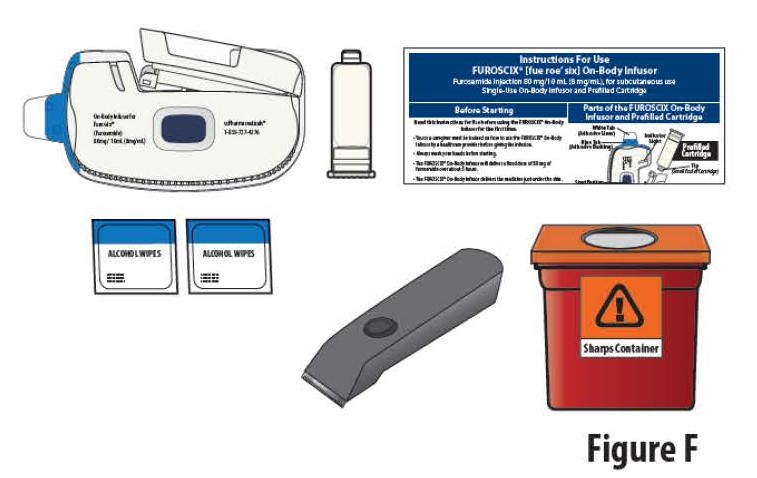
1.4Gather all materials needed for your injection, on a clean, well-lit work surface (Figure F):
- On-body Infusor and prefilled cartridge (Included)
- Instructions For Use (Included)
- Alcohol wipes (Included)
- Clippers (if needed) (Not included)
- Sharps disposal container (Not included)
STEP 2 Check On-Body Infusor and Prefilled Cartridge
2.1 Check the on-body Infusor and prefilled cartridge for any damage (see Figure G).
Do notuse the on-body Infusor or prefilled cartridge if either item appears to be damaged.
Do nottouch the blue start button until the on-body Infusor is on the skin and you are ready to begin the infusion.
Do not touch or fully open the cartridge holder until you are ready to load the cartridge.
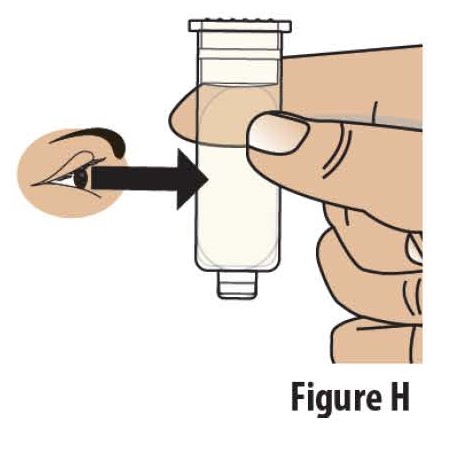
2.2Check the liquid medicine in the prefilled cartridge (see Figure H). The liquid in the prefilled cartridge should be clear to slightly yellow.
Do notuse the prefilled cartridge if the liquid is discolored or cloudy.
Call scPharmaceuticals at: 1-855-SCPHARMA (1-855-727-4276) or call your healthcare provider.
STEP 3 Load Prefilled Cartridge into the On-Body Infusor
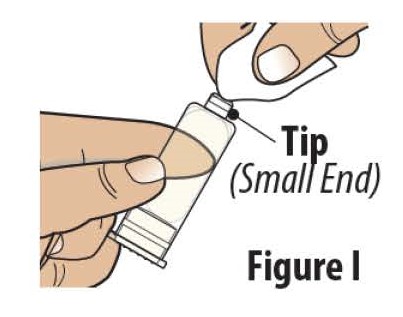
3.1 Clean the tip (small end) of the prefilled cartridge with an alcohol wipe (see Figure I).
Do notcontinue with the process of opening the cartridge holder all the way or loading the prefilled cartridge until you are ready to begin the infusion.
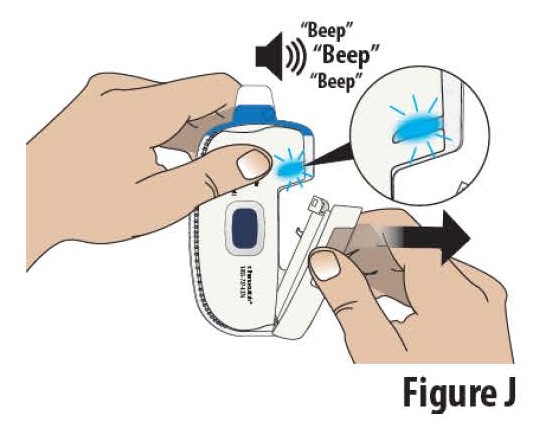
3.2Open the cartridge holder all the way to turn on the on-body Infusor. The on-body Infusor “beeps” and the indicator light will begin blinking blue (see Figure J).
Note: Complete steps 3.3 through 6.1 within 30 minutes of fully opening the cartridge holder or the Infusor will alarm and cannot restart.
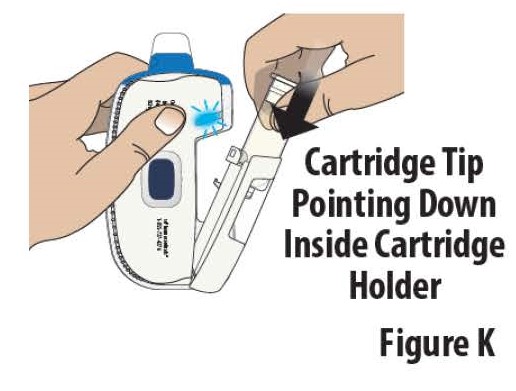
3.3 Insert the prefilled cartridge into the cartridge holder with the tip pointed down (see Figure K).
**Note:**The prefilled cartridge should slide into the cartridge holder easily. If it does not slide in easily, check the prefilled cartridge to make sure the tip is pointed down and try again.
Do not press the blue start button until on-body Infusor is applied to skin and you are ready to begin the infusion.
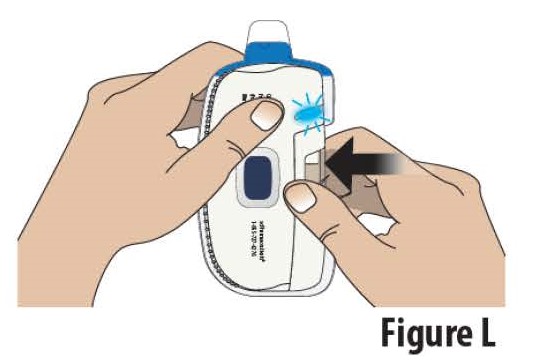
3.4Close the cartridge holder all the way until it is even with the rest of the on-body Infusor (see Figure L). You may hear a “click” sound when it is closed.
**Note:**If the indicator light blinks red and the on-body Infusor "beeps", the on-body Infusor has experienced an internal error.Do not use the on- body Infusor (see On-Body Infusor Alarm section).
Apply the On-Body Infusor
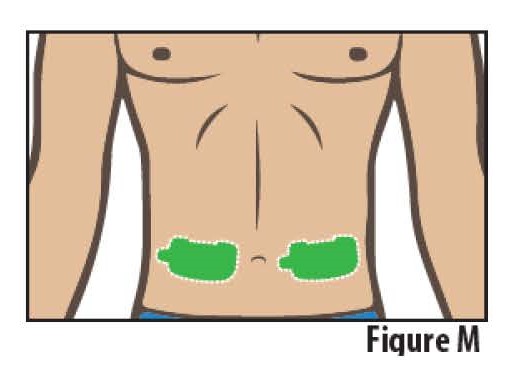
STEP 4 Select and Prepare Application Site
4.1Select one site on the stomach on either side of the belly button (navel). The site should be aflat area below the rib cage and above the belt line (see Figure M).
Do notselect a site where the skin is irritated or broken.
Do not apply lotions, oils or ointments to the adhesion area of the abdomen.
Do not select a site where belts, waistbands or other types of clothing may rub against, disturb, or dislodge the on-body Infusor.
Note: Rotate application site (side of the stomach) each time if told by your healthcare provider to repeat dose.
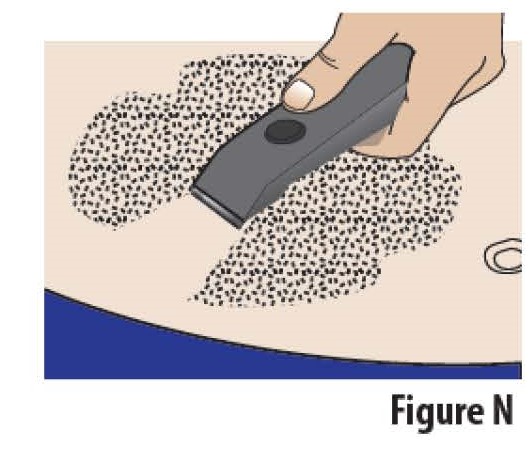
4.2The site should be hairless or nearly hairless. If needed, remove excess hair by clipping or shaving the hair before applying the on-body Infusor (see Figure N).
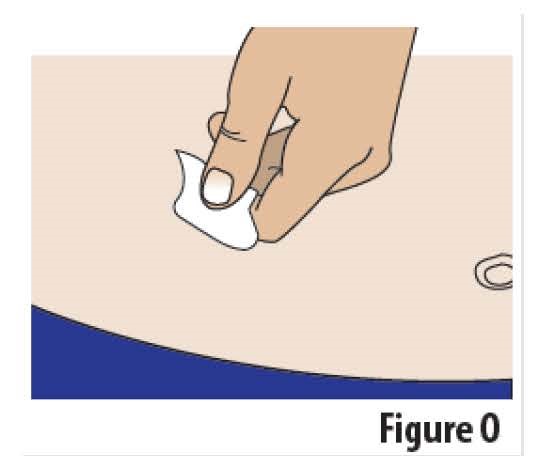
4.3 Wipe the skin where the on-body Infusor will be applied with an alcohol wipe and allow area to dry (see Figure O).
STEP 5 Apply the On-Body Infusor
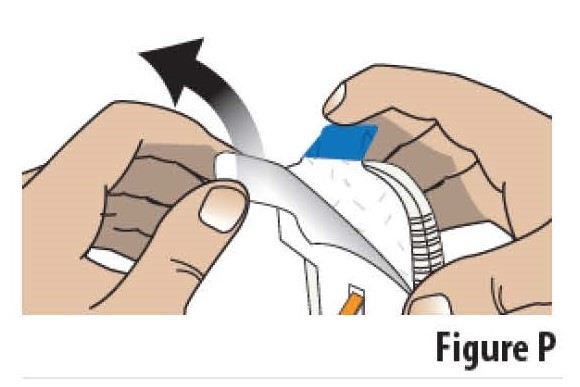
5.1Pick up the on-body Infusor. Peel away the adhesive liner by grasping the white tab and pulling it away from the blue tab/adhesive backing (see Figure P).
Do not touch the sticky (adhesive) part of the on-body Infusor with your fingers or let it touch any objects or surfaces before the Infusor is placed on the skin.
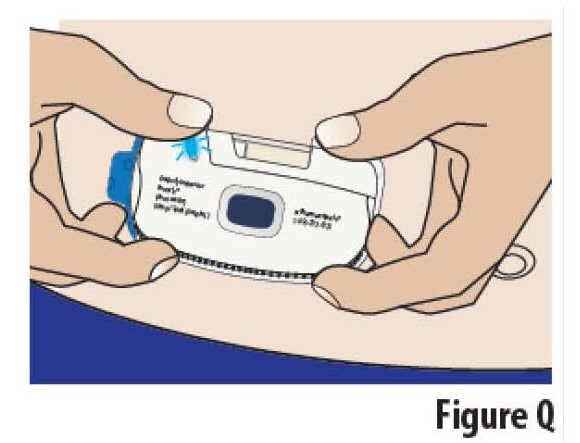
5.2 Apply the on-body Infusor to the chosen skin site while standing or sitting up straight.
Position the on-body Infusor so that the cartridge window and the indicator light can be seen during the infusion (see Figure Q).
Do not bend over when applying the on-body Infusor.
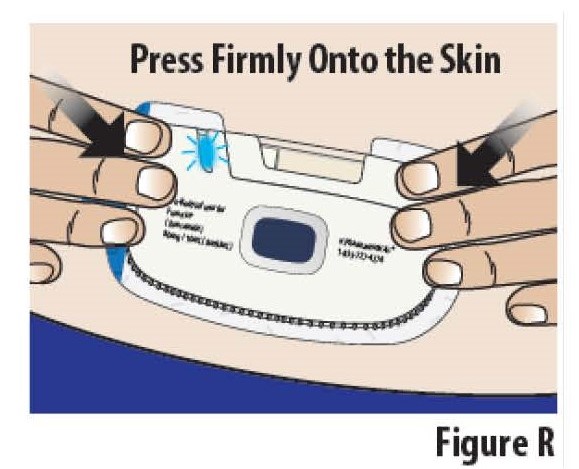
5.3Press the on-body Infusor firmly onto the skin and hold it for several seconds (see Figure R). Then rub your finger over the edges of the adhesive to get it to stick well.
Do not touch the blue start button until you are ready to start the infusion (see Step 6).
Do not remove and reapply on-body Infusor. The on-body Infusor adhesive might not work as well.
Start the Infusion
STEP 6 Start the Infusion
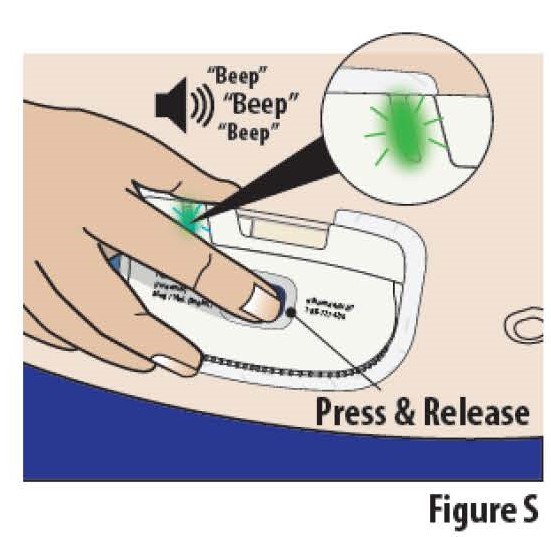
6.1Firmlypress and release the blue start button to begin the infusion (see Figure S).
The on-body Infusor will stick a very small needle just under the skin and begin delivering the medicine. The indicator light will start blinking green and the on-body Infusor "beeps", signifying that the infusion has begun.
**Note:**At the start of the infusion, you may also hear the on-body Infusor motor running for several seconds.
STEP 7 Allow Medicine to Deliver for 5 Hours
7.1Relax during the delivery period. The on-body Infusor should remain on the skin until the delivery is complete.
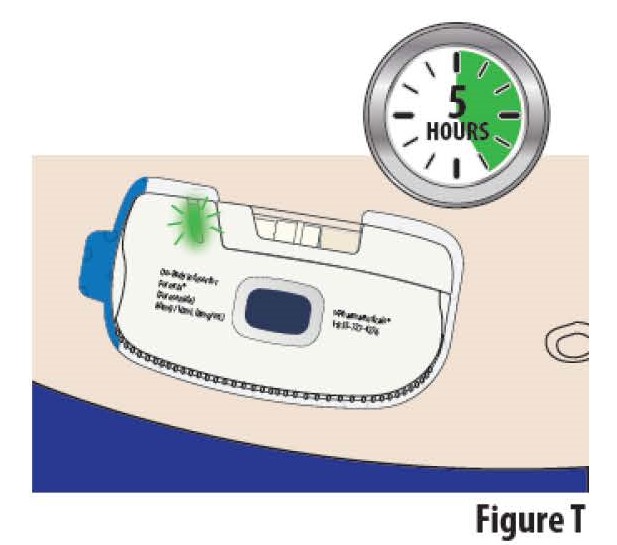
Do notexercise or take part in activities that might result in the on- body Infusor falling off. Take care not to bump the on-body Infusor while wearing it. It takes about 5 hours for the on-body Infusor to deliver all of the medicine. During the infusion, the indicator light will continue to blink green periodically, signifying that the infusion is running (see Figure T).
**Note:**If the on-body Infusor falls off or if the indicator light begins blinking red, call your healthcare provider right away (see On-Body Infusor Alarm section).Do not reapply or reuse the on-body Infusor.**Do not **apply a new on-body Infusor unless instructed to do so by your healthcare provider.
If You Have to Stop the Infusion Due to An Emergency, Do the Following:
- Remove the on-body Infusor from the skin (see Step 8). It will immediately stop infusing and the on-body Infusor will deactivate.Do notreuse the on-body Infusor. After the on-body Infusor is removed, it can no longer be used.
- Call your healthcare provider for further instructions.
Remove and Dispose of the On-Body Infusor
STEP 8 When Infusion is Complete, Remove the On-Body Infusor from the Skin
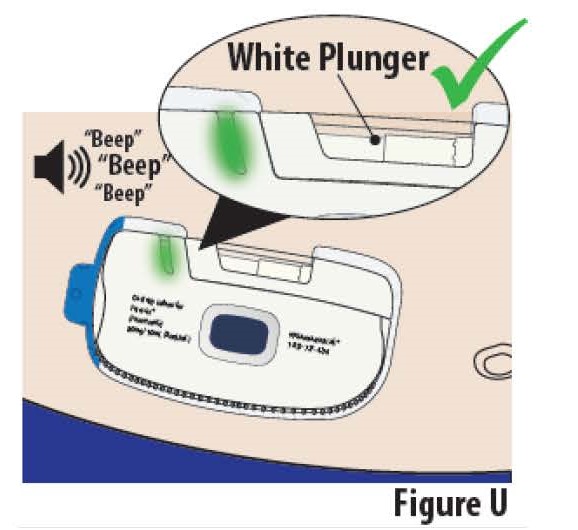
8.1The infusion is complete and the on-body Infusor is ready to be removed when (see Figure U):
- The indicator light turns solid green.
- The on-body Infusor "beeps".
- The white plunger rod fills the cartridge window all the way.
Note: If indicator light begins blinking red instead of green and the on- body Infusor “beeps”, call your healthcare provider (see On-Body Infusor Alarm section).
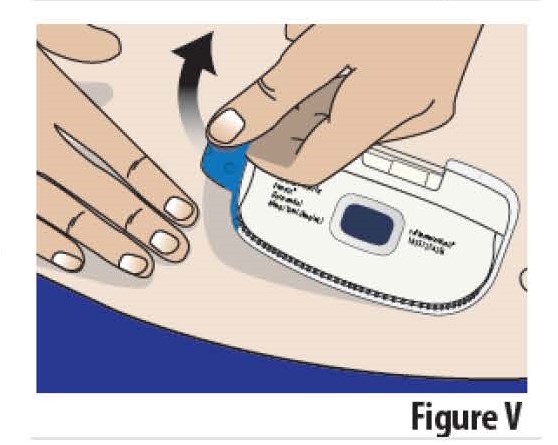
8.2 Peel the on-body Infusor off the skin by holding the skin down and pulling on the blue tab (see Figure V). When the on-body Infusor is removed, the indicator light will turn off, the needle cover will extend over the needle to protect against accidental needle sticks, and the on-body Infusor will turn off.
**Note:**You might have some discomfort after you remove the adhesive. This discomfort should quickly go away. However, some redness of the skin may remain.
8.3 Check the infusion site. If there is any bleeding use a cotton ball or apply a small adhesive bandage.
STEP 9 Throw Away (Dispose) of Used On-Body Infusor
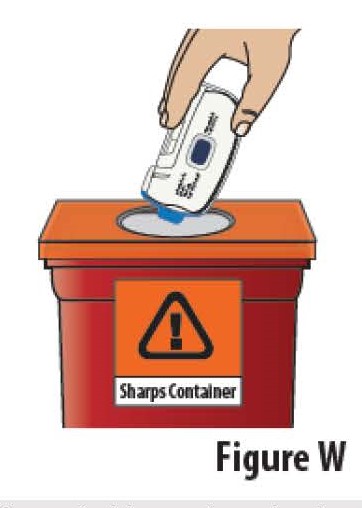
9.1Dispose of the used FUROSCIX On-Body Infusor and prefilled cartridge together into an FDA-cleared sharps container (see Figure W).
Do not attempt to remove the cartridge from the on-body Infusor; it cannot be removed.
Do not throw away the used on-body Infusor into household trash.
If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used Needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this.
Do not recycle the on-body Infusor or sharps disposal container or throw them into household trash.
**Important:**Always keep the sharps disposal container out of the reach of children.
For more information refer to: http://www.safeneedledisposal.org
Infusor Alarm
What happened?
The Indicator light blinks red and the on-body Infusor "beeps" (see Figure X).
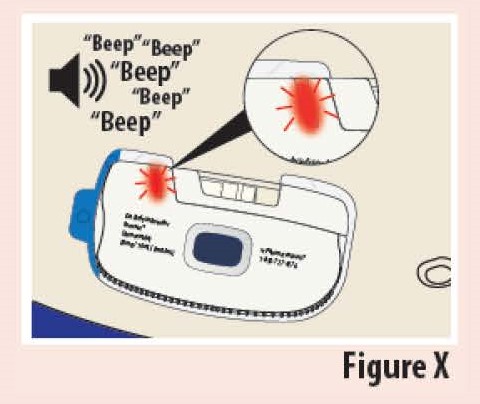
What does it mean?
There has been an internal error or the on-body Infusor came off the skin during the infusion.
What to do?
Stop using the on-body Infusor. If the on-body Infusor is on the skin, carefully remove it. Call your healthcare provider for further instructions.
Do notreapply or reuse the on-body Infusor. It can no longer be used.
Do not apply a new on-body Infusor unless instructed to do so by your healthcare provider.
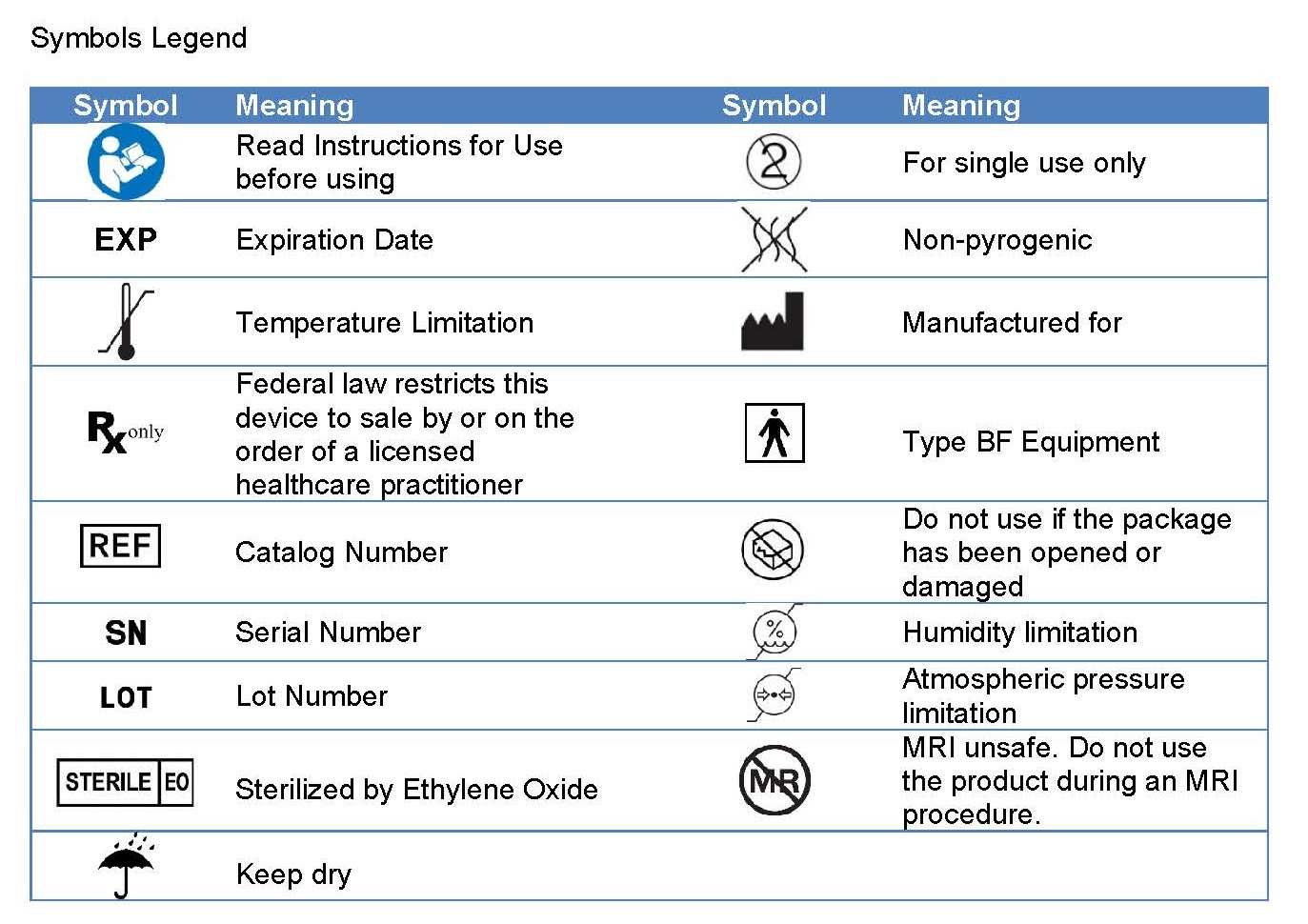
Symbols Legend
scPharmaceuticals, Inc.
25 Mall Road, Suite 203, Burlington, MA 01803
1-855-SCPHARMA (1-855-727-4276)
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Issued: 10/2023
Reg-0017 Rev 01
RECENT MAJOR CHANGES SECTION
RECENT MAJOR CHANGES
Indications and Usage ( 1)
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
FUROSCIX injection is a sterile, clear to slightly yellow, non-pyrogenic liquid supplied in a single-dose prefilled cartridge for subcutaneous infusion co-packaged with the On-body Infusor. Each single-use On-body Infusor with prefilled cartridge is designed to deliver 80 mg of FUROSCIX in 10 mL solution over 5-hours.
|
Carton containing one 80 mg/10 mL (8 mg/mL) prefilled cartridge co-packaged with one On-body Infusor |
NDC 71767-100-01 |
Store between 20°C and 25°C (68°F and 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F) [See USP Controlled Room Temperature]. Do not refrigerate or freeze.
Protect FUROSCIX from light. Do not remove the cartridge from carton until it is ready for use. Do not use if the solution is discolored or cloudy. Protect the On-body Infusor from water.
PATIENT COUNSELING INFORMATION
17 PATIENT COUNSELING INFORMATION
Advise the patient and/or caregiver to read the FDA-approved patient labeling [see FDA-approved Instructions for Use].
Advise the patient that they should not allow the On-body Infusor to come into contact with water or other fluids. Patients should check the device for alarms to ensure a complete dose is administered [see Warnings and Precautions ( 5.5)] .
Fluid, Electrolyte, and Metabolic Abnormalities
Advise patients that they may experience symptoms from excessive fluid and/or electrolyte losses. The postural hypotension that sometimes occurs can usually be managed by getting up slowly. Potassium supplements and/or dietary measures may be needed to control or avoid hypokalemia [see Warnings and Precautions ( 5.1)] .
Advise patients that furosemide may increase blood glucose levels and thereby affect urine glucose tests [see Warnings and Precautions ( 5.1)].
Photosensitivity
The skin of some patients may be more sensitive to the effects of sunlight while taking furosemide [see Adverse Reactions ( 6)] .
Advise hypertensive patients to avoid medications that may increase blood pressure, including over-the-counter products for appetite suppression and cold symptoms [see Drug Interactions ( 7.1)] .
For more information about FUROSCIX, go to www.FUROSCIX.com or call 1-855-SCPHARMA (1-855-727-4276)
scPharmaceuticals, Inc.
FUROSCIX ® (furosemide injection 80 mg/10 mL) for subcutaneous use
Manufactured for:
scPharmaceuticals, Inc.,
25 Mall Road, Suite 203,
Burlington, MA 01803
Patent Protected: www.scpharmaceuticals.com/patents
FUROSCIX ® is a mark of scPharmaceuticals Inc.
Reg-0018 Rev 02
