Products (2)
Levalbuterol Hydrochloride
76204-002
NDA020837
NDA authorized generic (C73605)
RESPIRATORY (INHALATION)
December 21, 2012
Levalbuterol Hydrochloride
76204-003
NDA020837
NDA authorized generic (C73605)
RESPIRATORY (INHALATION)
December 21, 2012
Drug Labeling Information
SPL PATIENT PACKAGE INSERT SECTION
Patient's Instructions for Use
Levalbuterol HCl
Inhalation Solution
0.63 mg2, 1.25 mg**2**
** 3 mL Unit-Dose Vials**
Read complete instructions carefully before using.
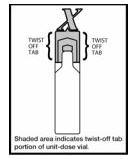
Figure 1
1.
Open the foil pouch by tearing on the serrated edge along the seam of the pouch. Remove one unit-dose vial for immediate use. Keep the rest of the unused unit-dose vials in the foil pouch to protect them from light.
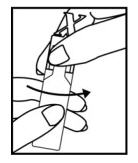
Figure 2
2.
Hold the unit-dose vial in your hands as shown in**Figure 2**.
3.
Ensure your thumb and finger cover the twist-off tabs below the "X" top (**Figure 2**).
4.
Twist the body of the unit-dose vial while holding the top firmly between your thumb and finger to open the vial.
5.
Discard the top and squeeze the entire contents into the nebulizer reservoir.
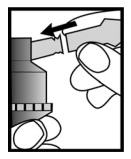
Figure 3
6.
Connect the nebulizer reservoir to the mouthpiece or face mask (**Figure 3**).
7.
Connect the nebulizer to the compressor.
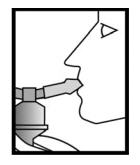
Figure 4
8.
Sit in a comfortable, upright position. Place the mouthpiece in your mouth (**Figure 4**) (or put on the face mask) and turn on the compressor.
9.
Breathe as calmly, deeply, and evenly as possible until no more mist is formed in the nebulizer reservoir (about 5 to 15 minutes). At this point, the treatment is finished.
10.
Clean the nebulizer (see manufacturer's instructions).
Note: Levalbuterol HCl Inhalation Solution should be used in a nebulizer only under the direction of a physician. More frequent administration or higher doses are not recommended without first discussing with your doctor. This solution should not be injected or administered orally. Protect from light and excessive heat. Store in the protective foil pouch at 20-25°C (68-77°F) [see USP Controlled Room Temperature]. Keep unopened vials in the foil pouch. Once the foil pouch is opened, the vials should be used within 2 weeks. Vials removed from the pouch, if not used immediately, should be protected from light and used within 1 week. Discard any vial if the solution is not colorless.
The safety and effectiveness of Levalbuterol HCl Inhalation Solution have not been determined when one or more drugs are mixed with it in a nebulizer. Check with your doctor before mixing any medications in your nebulizer.
Manufactured for:
Ritedose Pharmaceuticals, LLC
Columbia, SC 29203 For customer service, call 1-888-394-7377.
To report adverse events, call 1-877-737-7226.
For medical information, call 1-800-739-0565.
September 2012
901721R00
2
**Potency expressed as levalbuterol**
