Desoximetasone
Desoximetasone Cream USP, 0.25%
f30b6fcc-43bc-4d65-9c3b-8d6ca780a024
HUMAN PRESCRIPTION DRUG LABEL
Apr 1, 2022
Proficient Rx LP
DUNS: 079196022
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Desoximetasone
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
Drug Labeling Information
INDICATIONS & USAGE SECTION
INDICATIONS AND USAGE
Desoximetasone cream USP, 0.25% is indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses.
CONTRAINDICATIONS SECTION
CONTRAINDICATIONS
Desoximetasone cream USP, 0.25% is contraindicated in those patients with a history of hypersensitivity to any of the components of the preparation.
SPL UNCLASSIFIED SECTION
Rx Only
For Topical Use Only.
Not for Oral, Ophthalmic, or Intravaginal Use.
DESCRIPTION SECTION
DESCRIPTION
Desoximetasone cream USP, 0.25% contains the active synthetic corticosteroid desoximetasone, USP. The topical corticosteroids constitute a class of primarily synthetic steroids used as anti-inflammatory and antipruritic agents.
Each gram of desoximetasone cream USP, 0.25% contains 2.5 mg of desoximetasone, USP in an emollient cream base consisting of cetostearyl alcohol, edetate disodium, isopropyl myristate, lanolin alcohol, mineral oil, purified water, and white petrolatum.
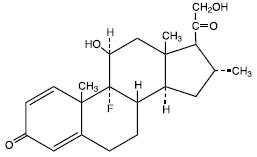
The chemical name of desoximetasone, USP is Pregna-1, 4-diene-3, 20-dione, 9-fluoro-11, 21-dihydroxy-16-methyl-,(11β,16α)-. Desoximetasone, USP has the molecular formula C22H29FO4 and a molecular weight of 376.47. The CAS Registry Number is 382-67-2.
The structural formula is:
CLINICAL PHARMACOLOGY SECTION
CLINICAL PHARMACOLOGY
Topical corticosteroids share anti-inflammatory, antipruritic and vasoconstrictive actions.
The mechanism of anti-inflammatory activity of the topical corticosteroids is unclear. Various laboratory methods, including vasoconstrictor assays, are used to compare and predict potencies and/or clinical efficacies of the topical corticosteroids. There is some evidence to suggest that a recognizable correlation exists between vasoconstrictor potency and therapeutic efficacy in man.
Pharmacokinetics
The extent of percutaneous absorption of topical corticosteroids is determined by many factors including the vehicle, the integrity of the epidermal barrier, and the use of occlusive dressings.
Topical corticosteroids can be absorbed from normal intact skin. Inflammation and/or other disease processes in the skin increase percutaneous absorption. Occlusive dressings substantially increase the percutaneous absorption of topical corticosteroids. Thus, occlusive dressings may be a valuable therapeutic adjunct for treatment of resistant dermatoses.
Once absorbed through the skin, topical corticosteroids are handled through pharmacokinetic pathways similar to systemically administered corticosteroids. Corticosteroids are bound to plasma proteins in varying degrees. Corticosteroids are metabolized primarily in the liver and are then excreted by the kidneys. Some of the topical corticosteroids and their metabolites are also excreted into the bile.
Pharmacokinetic studies in men with desoximetasone cream USP, 0.25% with tagged desoximetasone showed a total of 5.2% ± 2.9% excretion in urine (4.1% ± 2.3%) and feces (1.1% ± 0.6%) and no detectable level (limit of sensitivity: 0.005 mcg/mL) in the blood when it was applied topically on the back followed by occlusion for 24 hours. Seven days after application, no further radioactivity was detected in urine or feces. The half-life of the material was 15 ± 2 hours (for urine) and 17 ± 2 hours (for feces) between the third and fifth trial day. Studies with other similarly structured steroids have shown that predominant metabolite reaction occurs through conjugation to form the glucuronide and sulfate ester.
WARNINGS SECTION
WARNINGS
Keep out of reach of children.
