SERYNTH FAST RELIEF NASAL
84010-117
35c90ed6-8b07-9507-e063-6394a90a6799
HUMAN OTC DRUG LABEL
May 23, 2025
Jiangxi Hemei Pharmaceutical Co., Ltd
DUNS: 724892056
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Naphazoline HCL 0.05% FAST RELIEF NASAL
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
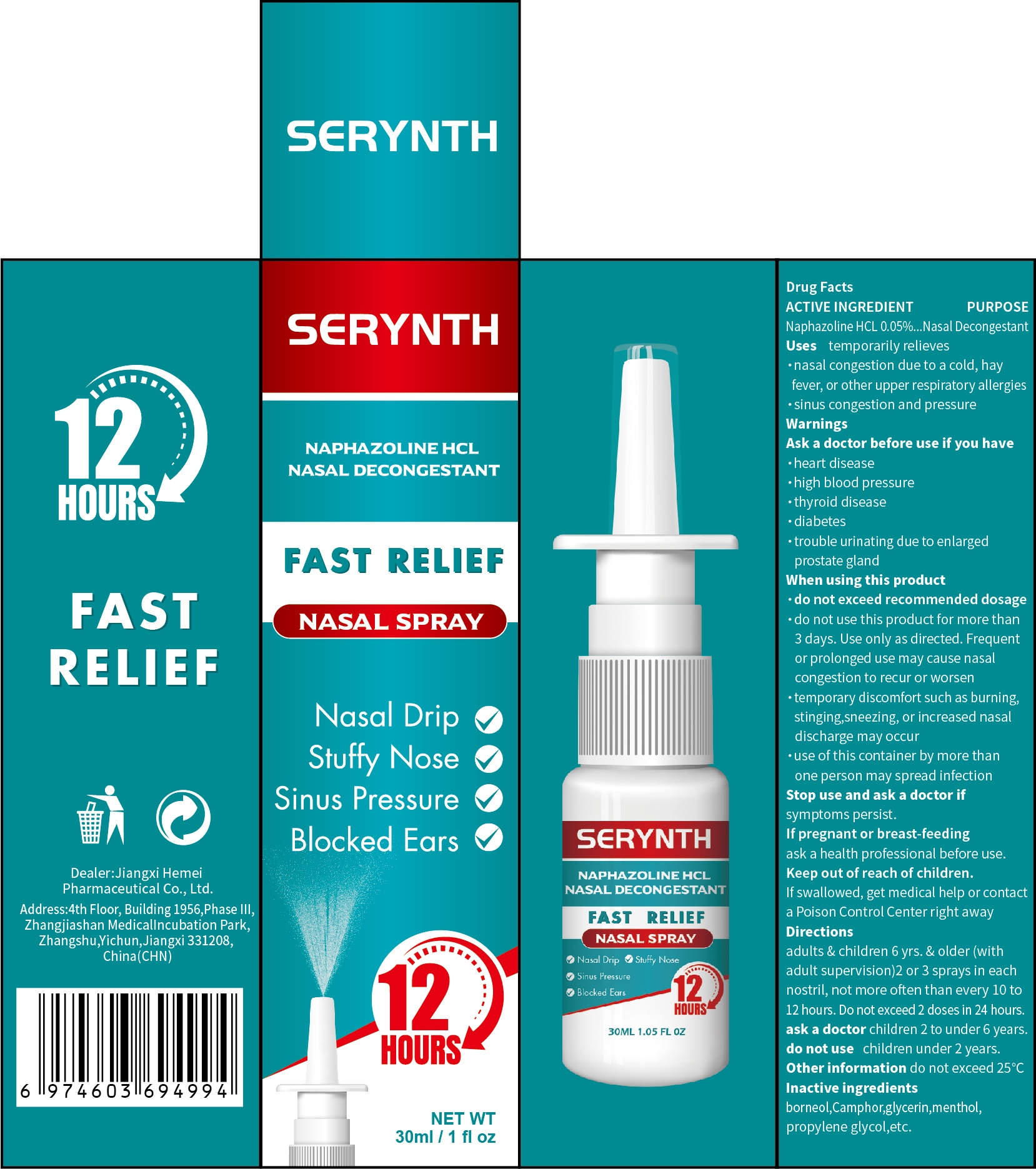
INDICATIONS & USAGE SECTION
Use
temporarily relieves
·nasal congestion due to a cold, hay fever, or other upper respiratory allergies
·sinus congestion and pressure
OTC - ACTIVE INGREDIENT SECTION
Active Ingredient
Naphazoline HCL 0.05%.
OTC - PURPOSE SECTION
Purpose
Nasal Decongestant
OTC - DO NOT USE SECTION
Do not use
·do not exceed recommended dosage
·do not use this product for more than 3 days. Use only as directed. Frequent or prolonged use may cause nasal congestion to recur or worsen
·temporary discomfort such as burning, stinging,sneezing, or increased
nasaldischarge may occur
·use of this container by more than one person may spread infection
do not use children under 2 years.
WARNINGS SECTION
Warnings
Ask a doctor before use if you have
·heart disease
·high blood pressure
·thyroid disease
·diabetes
·trouble urinating due to enlarged prostate gland
OTC - WHEN USING SECTION
When Using
·do not exceed recommended dosage
·do not use this product for more than 3 days. Use only as directed. Frequent or prolonged use may cause nasal congestion to recur or worsen
·temporary discomfort such as burning, stinging,sneezing, or increased
nasaldischarge may occur
·use of this container by more than one person may spread infection
OTC - STOP USE SECTION
Stop Use
Stop use and ask a doctor if symptoms persist.
OTC - ASK DOCTOR SECTION
Ask Doctor
If pregnant or breast-feeding, ask a health professional before use.
children 2 to under 6 years.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep Out Of Reach Of Children
If swallowed,get medical help or contact a Poison Control Center right away
DOSAGE & ADMINISTRATION SECTION
Directions
adults&children 6 years &older(with adult supervision ) 2 or 3 sprays in each nostril, not more often than every 10 to 12 hours.Do not exceed 2 doses in 24 hours
STORAGE AND HANDLING SECTION
Other information
do not exceed 25°C.
INACTIVE INGREDIENT SECTION
Inactive ingredients
borneol,Camphor,glycerin,menthol,propylene glycol,etc.
