Acid Gone Antacid
Extra Strength Acid Gone Antacid 100 Chewable Tablets Drug Facts
4498104a-e41d-41d9-9aed-01904d91af87
HUMAN OTC DRUG LABEL
Sep 19, 2025
Major Pharmaceuticals
DUNS: 191427277
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
aluminum hydroxide and magnesium carbonate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
Drug Labeling Information
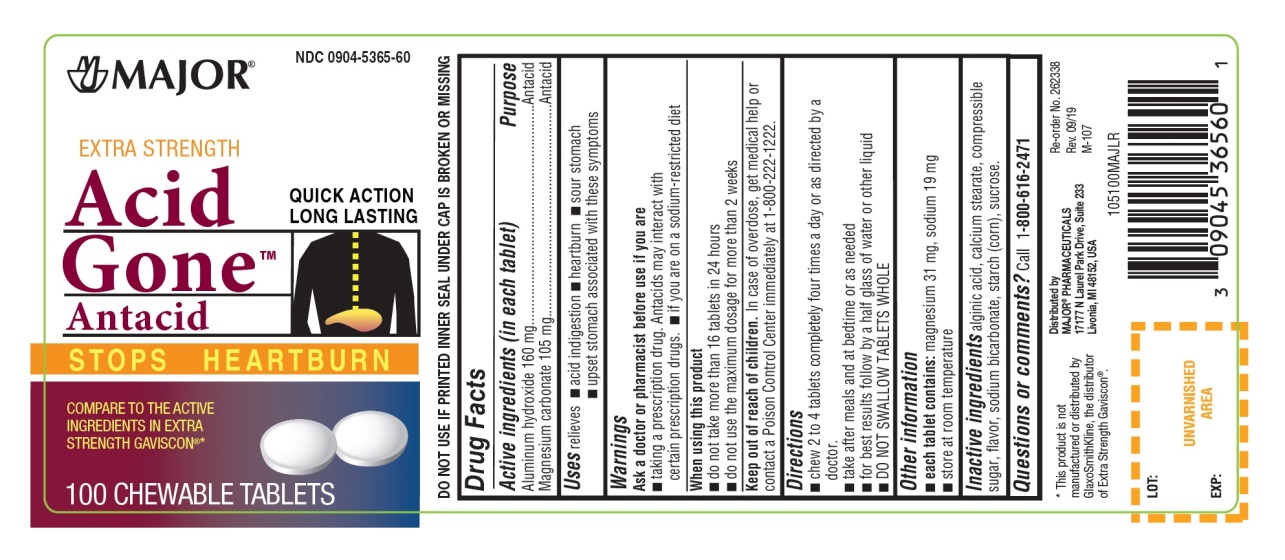
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Principal Display Panel
NDC 0904-5365-60
EXTRA STRENGTH
Acid Gone™
Antacid
STOPS HEARTBURN
QUICK ACTION
LONG LASTING
COMPARE TO THE ACTIVE INGREDIENT IN EXTRA STRENGTH GAVISCON ®*
100 CHEWABLE TABLETS
Distributed by:
MAJOR**®**PHARMACEUTICALS
17177 N Laurel Park Drive, Suite 233
Livonia, MI 48150, USA
*This product is not manufactured or distributed by GlaxoSmithKline, the distributor of Extra Strength Gaviscon ®
INDICATIONS & USAGE SECTION
Uses
relieves
- acid indigestion
- heartburn
- sour stomach
- upset stomach associated with these symptoms
INACTIVE INGREDIENT SECTION
Inactive ingredients
alginic acid, calcium stearate, compressible sugar, flavor, sodium bicarbonate, starch(corn), sucrose.
OTC - QUESTIONS SECTION
Questions or comments?
call 1-800-616-2471
OTC - ACTIVE INGREDIENT SECTION
Active ingredients (in each tablet)
Aluminum hydroxide 160mg
Magnesium carbonate 105mg
OTC - PURPOSE SECTION
Purpose
Antacid
WARNINGS SECTION
Warnings
Ask a doctor or pharmacist before use if you are
- taking a prescription drug. Antacids may interact with certain prescription drugs.
- if you are on a sodium-restricted diet
When using this product
- do not take more than 16 tablets in 24 hours
- do not use the maximum dosage for more than 2 weeks
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center immediately at 1-800-222-1222.
DOSAGE & ADMINISTRATION SECTION
Directions
- chew 2-4 tablets four times a day or as directed by a doctor
- take after meals and at bedtime or as needed
- for best results follow by a half glass of water or other liquid
- DO NOT SWALLOW TABLET WHOLE
SPL UNCLASSIFIED SECTION
Other information
- Each tablet contains**:**sodium 19 mg, magnesium 31mg
- Store at room temperature
