Alprazolam
These highlights do not include all the information needed to use ALPRAZOLAM TABLETS safely and effectively. See full prescribing information for ALPRAZOLAM TABLETS. ALPRAZOLAM tablets, for oral use, CIV Initial U.S. Approval: 1981
68d8b8d1-00a4-42c4-a796-90211fee1a6d
HUMAN PRESCRIPTION DRUG LABEL
Aug 20, 2025
Bryant Ranch Prepack
DUNS: 171714327
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Alprazolam
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
Drug Labeling Information
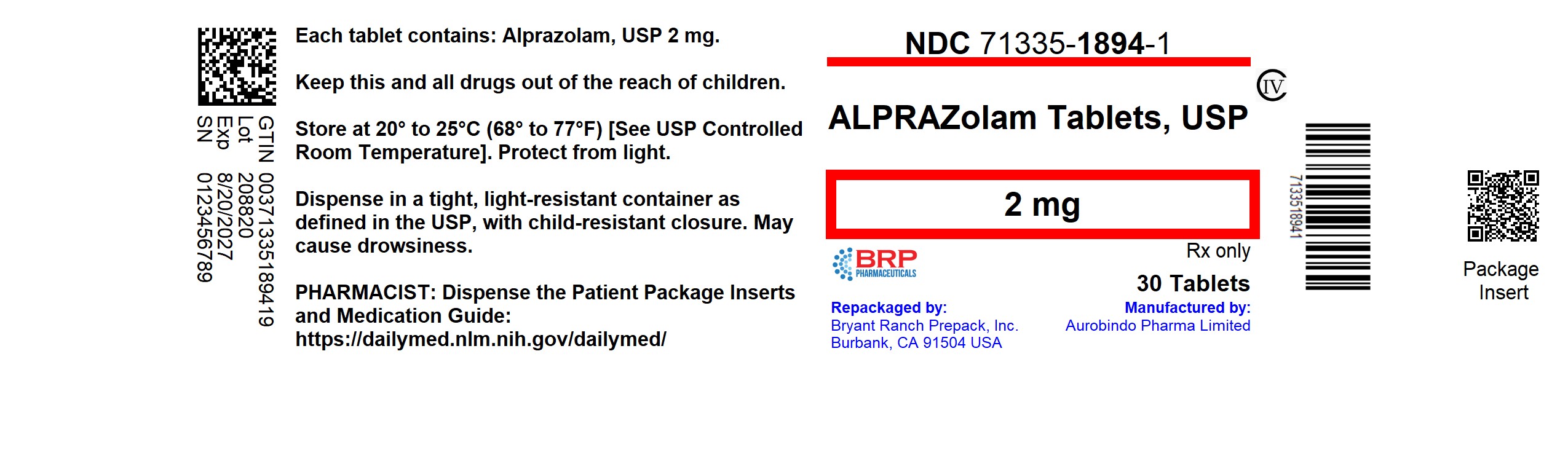
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Alprazolam 2mg(CIV) Tablet
DESCRIPTION SECTION
11 DESCRIPTION
Alprazolam tablets, USP contain alprazolam which is a triazolo analog of the 1,4 benzodiazepine class of central nervous system-active compounds.
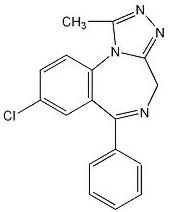
The chemical name of alprazolam is 8-Chloro-1-methyl-6-phenyl-4H-s-triazolo [4,3-α] [1,4] benzodiazepine.
The structural formula is:
Alprazolam USP is a white to off-white, crystalline powder, which is soluble in methanol or ethanol but which has no appreciable solubility in water at physiological pH.
Each alprazolam tablet USP, for oral administration, contains 0.25 mg, 0.5 mg, 1 mg, or 2 mg of alprazolam USP.
Inactive ingredients: colloidal silicon dioxide, corn starch, docusate sodium 85% with sodium benzoate 15%, lactose monohydrate, magnesium stearate, and microcrystalline cellulose. In addition, the 0.5 mg tablet contains FD&C Yellow # 6 aluminum lake and the 1 mg tablet contains FD&C Blue No. 2 lake.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Alprazolam Tablets USP, 2 mg are white, oblong, uncoated tablets with three breaklines on both sides debossed with ‘2’ and ‘1’ on either sides of the center breakline and ‘Y’ on the other side.
NDC: 71335-1894-1: 30 Tablets in a BOTTLE
NDC: 71335-1894-2: 60 Tablets in a BOTTLE
NDC: 71335-1894-3: 90 Tablets in a BOTTLE
NDC: 71335-1894-4: 100 Tablets in a BOTTLE
NDC: 71335-1894-5: 10 Tablets in a BOTTLE
NDC: 71335-1894-6: 120 Tablets in a BOTTLE
NDC: 71335-1894-7: 40 Tablets in a BOTTLE
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Repackaged/Relabeled by:
Bryant Ranch Prepack, Inc.
Burbank, CA 91504
