Zanosar
Zanosar (Streptozocin) powder, for injection
Approved
Approval ID
f31d3bff-6fc6-c35f-e053-2995a90a596b
Product Type
HUMAN PRESCRIPTION DRUG LABEL
Effective Date
Apr 3, 2023
Manufacturers
FDA
ESTEVE PHARMACEUTICALS, S.A.
DUNS: 460023922
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Streptozocin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
NDC Product Code68118-100
Product Classification
G
Generic Name
Streptozocin
Product Specifications
Route of AdministrationINTRAVENOUS
Effective DateApril 3, 2023
FDA Product Classification
INGREDIENTS (3)
ANHYDROUS CITRIC ACIDInactive
Code: XF417D3PSL
Classification: IACT
SODIUM HYDROXIDEInactive
Code: 55X04QC32I
Classification: IACT
STREPTOZOCINActive
Quantity: 1 g in 10 mL
Code: 5W494URQ81
Classification: ACTIB
Drug Labeling Information
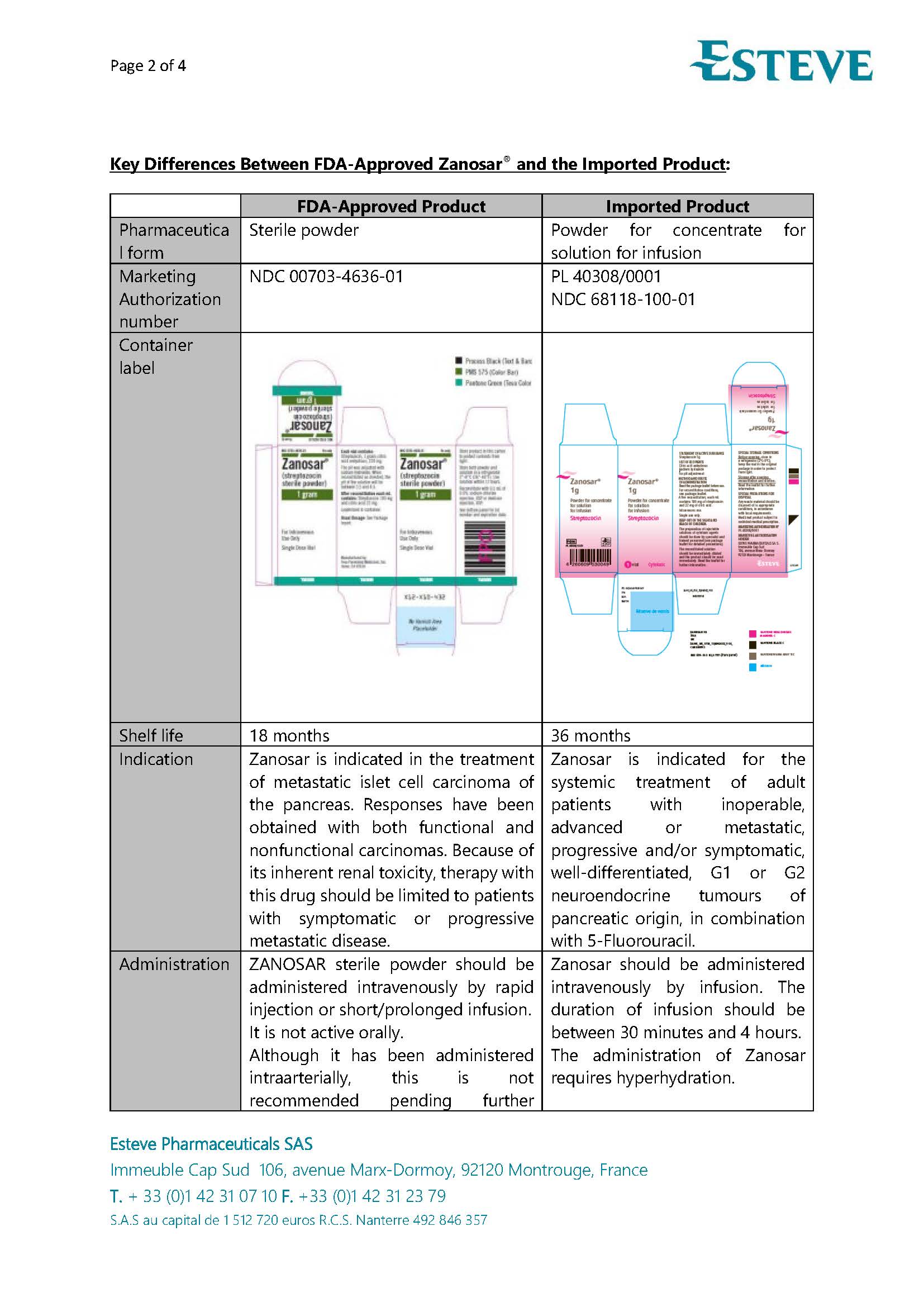
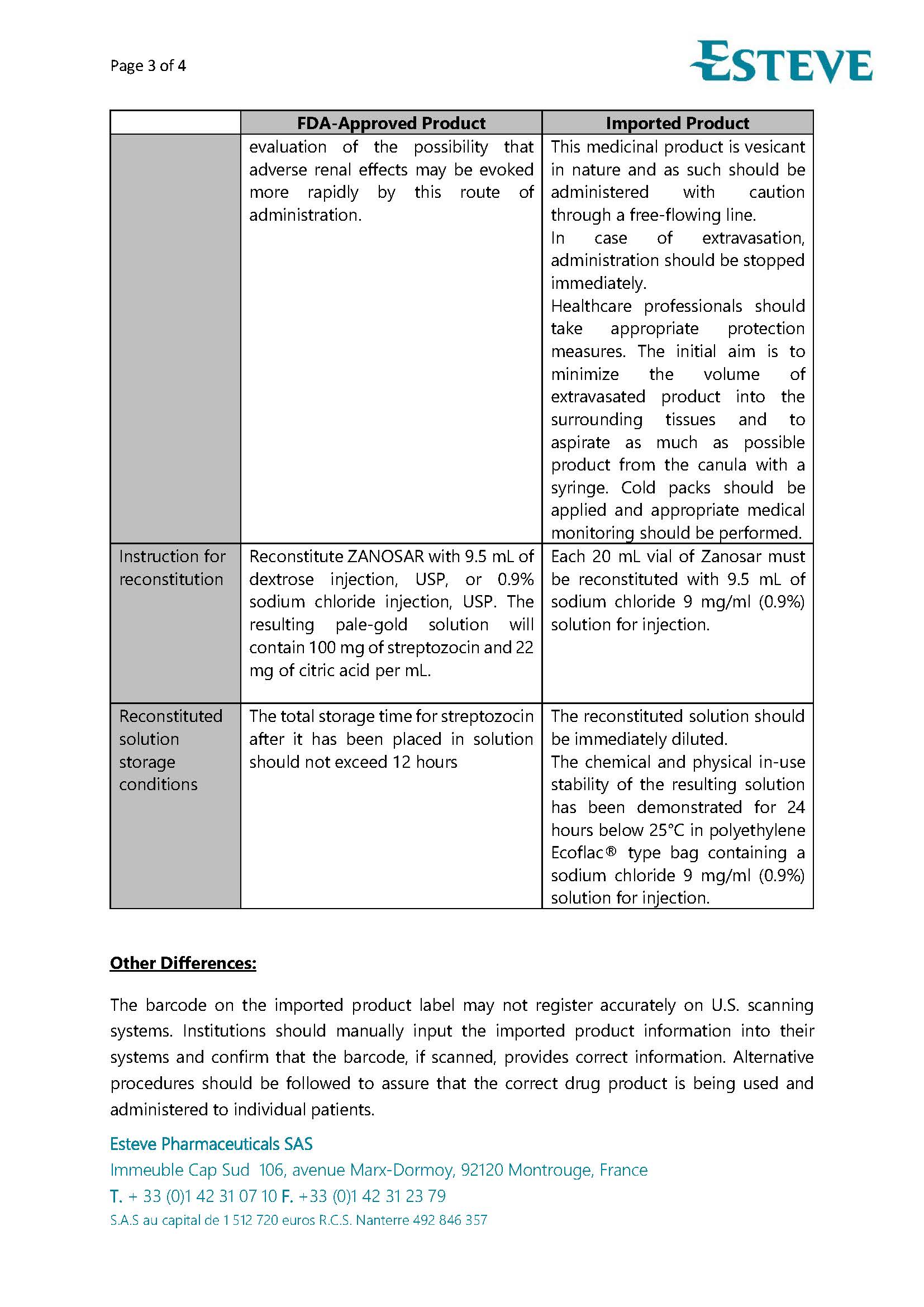
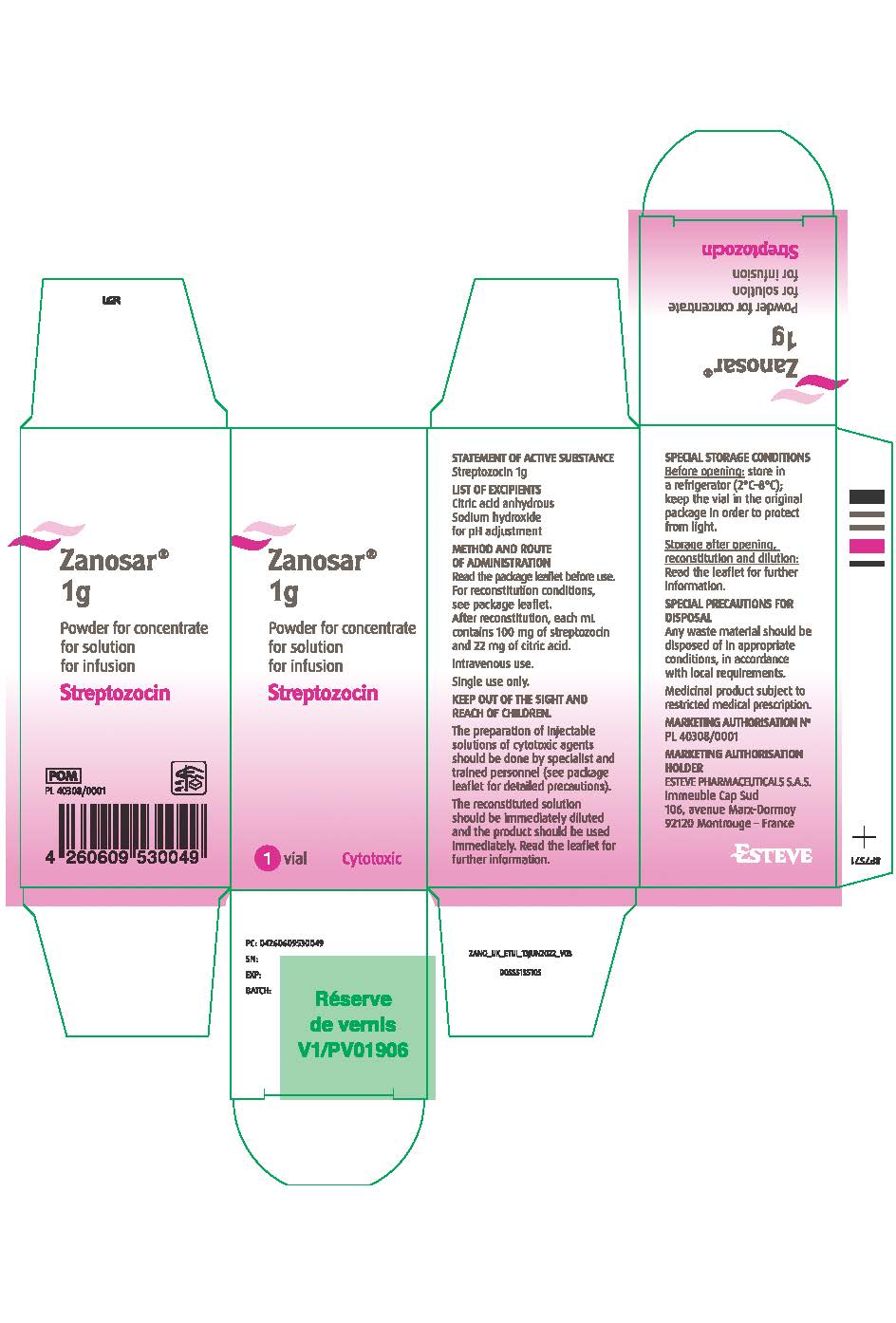
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
LOINC: 51945-4Updated: 5/9/2025
Carton Label
HOW SUPPLIED SECTION
LOINC: 34069-5Updated: 5/9/2025
How supplied
NDC 68118-100-01: Each vial contains 1g Streptozocin Powder for concentrate for solution for infusion. Each carton contains 1 vial.
HEALTH CARE PROVIDER LETTER SECTION
LOINC: 71744-7Updated: 5/9/2025
Dear Health Care Provider Letter