Benznidazole
These highlights do not include all the information needed to use BENZNIDAZOLE TABLETS safely and effectively. See full prescribing information for BENZNIDAZOLE TABLETS. BENZNIDAZOLE tablets, for oral use Initial U.S. Approval: 2017
8983d6a0-f63f-4f8e-bba4-38223f39e29b
HUMAN PRESCRIPTION DRUG LABEL
Sep 15, 2025
Exeltis USA, Inc.
DUNS: 071170534
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
benznidazole
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
benznidazole
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 12.5 mg CARTON
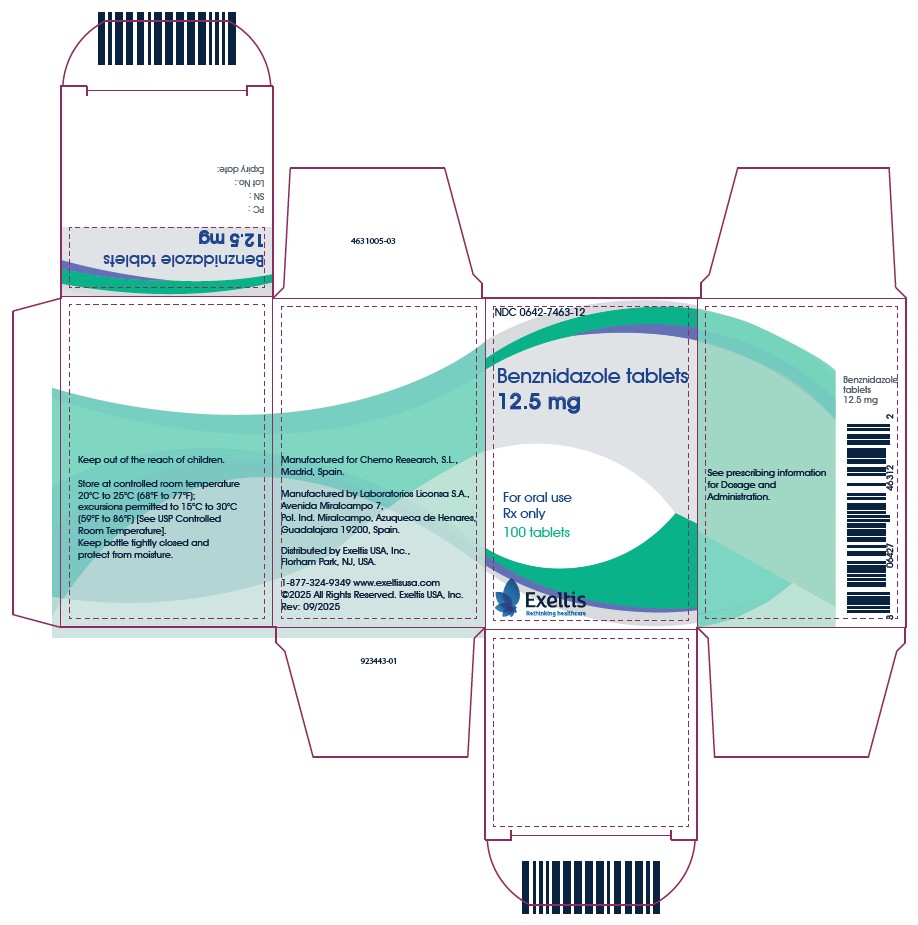
NDC 0642-7463-12
Benznidazole tablets
12.5 mg
For oral use
Rx only
100 tablets
Exeltis
Rethinking healthcare
Keep out of the reach of children.
Store at controlled room temperature 20°C to 25°C (68°F to 77°F); excursions
permitted to 15°C to 30°C (59°F to 86°F) [See USP Controlled Room Temperature].
Keep bottle tightly closed and protect from moisture.
Manufactured for Chemo Research, S.L.,
Madrid, Spain.
Manufactured by Laboratorios Liconsa S.A.,
Avenida Miralcampo 7,
Pol. Ind. Miralcampo, Azuqueca de Henares,
Guadalajara 19200, Spain.
Distributed by Exeltis USA, Inc.,
Florham Park, NJ, USA.
1-877-324-9349 www.exeltisusa.com
©2025 All Rights Reserved. Exeltis USA, Inc.
Rev: 09/2025
See prescribing information
for Dosage and
Administration.
Benznidazole
tablets
12.5 mg
Benznidazole tablets
12.5 mg
Lot No.:
Expiry date:
DESCRIPTION SECTION
11 DESCRIPTION
Benznidazole Tablets contain benznidazole, a nitroimidazole antimicrobial. The chemical name of benznidazole is N-benzyl-2-(2-nitro-1H-imidazol-1-yl) acetamide. The empirical formula is C 12H 12N 4O 3 and the molecular weight is 260.246 g/mol. The structural formula is:
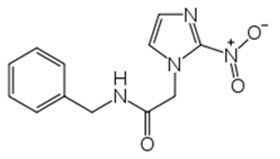
Figure 1: Benznidazole Structure
Benznidazole is a yellowish, practically crystalline powder that is practically insoluble in water, sparingly soluble in acetone and ethanol, and slightly soluble in methanol.
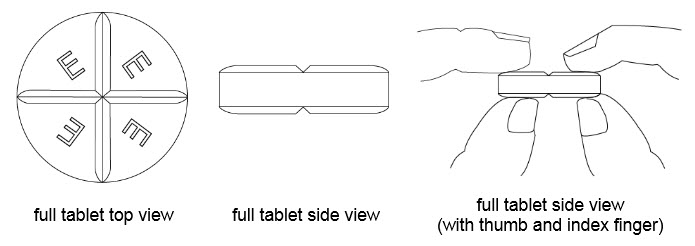
Benznidazole Tablets are white round tablets each containing 12.5 mg or 100 mg of benznidazole, for oral use. The 100 mg white tablets are round and functionally scored twice as a cross on both sides debossed with “E” on one side of each quarter portion. The 12.5 mg white tablets are round and unscored debossed with “E” on one side.
The inactive ingredients are as follows: magnesium stearate, NF; microcrystalline cellulose, NF; monohydrate lactose, NF; pre-gelatinized corn starch, NF; and sodium croscarmellose, NF.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling ( Instructions for Use).
Embryo-Fetal Toxicity
Advise pregnant women and females of reproductive potential that exposure to Benznidazole Tablets during pregnancy can result in fetal harm.
Advise females to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions ( 5.2) and Use in Specific Populations ( 8.1)] .
Advise females of reproductive potential to use effective contraception while taking
Benznidazole Tablets and for 5 days after the last dose [see Use in Specific Populations ( 8.3)] .
Lactation
Advise women not to breastfeed during treatment with Benznidazole Tablets [see Use in Specific Populations ( 8.2)] .
Infertility
Advise males of reproductive potential that Benznidazole Tablets may impair fertility [see Use in Specific Populations ( 8.3) and Nonclinical Toxicology ( 13.1)] .
Important Administration Instructions
Advise patients and parents/caregivers of pediatric patients taking Benznidazole Tablets that:
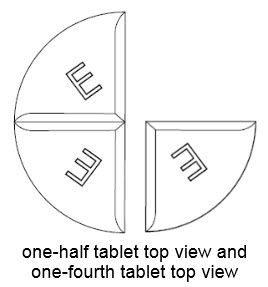
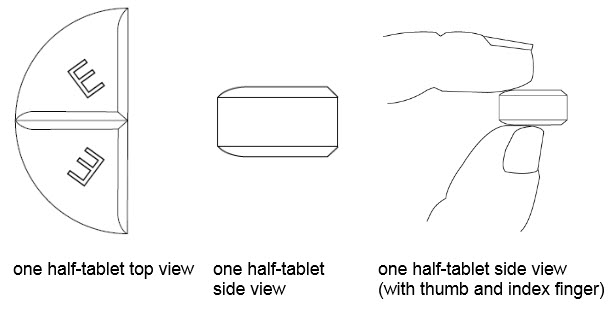
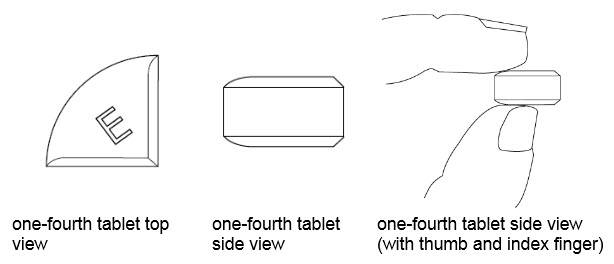
•Benznidazole Tablets 100 mg are functionally scored tablets which can be split into one-half (50 mg) or one-quarter (25 mg) at the scored lines to provide doses less than 100 mg.
•Benznidazole Tablets 12.5 mg and 100 mg (whole or split) can be made into a slurry in a specified volume of water for the pediatric population [see Dosage and Administration ( 2.3)] .
Hypersensitivity Skin Reactions
Advise patients that serious skin reactions can occur with Benznidazole Tablets. In case of skin reactions, presenting with additional symptoms of systemic involvement such as lymphadenopathy, fever and/or purpura, discontinuation of treatment is necessary.
Central and Peripheral Nervous System Effects
Advise patients that treatment can potentially cause paresthesia or symptoms of peripheral neuropathy. In cases where neurological symptoms occur, immediate discontinuation of treatment is recommended.
Hematological Manifestations of Bone Marrow Depression
Advise patients that there have been hematological manifestations of bone marrow depression, such as anemia and leukopenia, which are reversible, and normalized after treatment discontinuation.
Manufactured for Chemo Research, S.L.
Madrid, Spain
Manufactured by Laboratorios Liconsa S.A.
Guadalajara, Spain
Distributed by:

Exeltis USA, Inc.
Florham Park, NJ 07932
SPL PATIENT PACKAGE INSERT SECTION
INSTRUCTIONS FOR USE
BENZNIDAZOLE
tablets, for oral use
Read this Instructions for Use before you start taking BENZNIDAZOLE and each time you get a refill. There may be new information. This information does not take the place of talking to your doctor about your medical condition or treatment.
Note:
• Your doctor may need to change your dose of BENZNIDAZOLE during treatment as needed.
• BENZNIDAZOLE 100 mg tablets can be taken whole or broken at scored lines.
• BENZNIDAZOLE 100 mg tablets are marked with scored lines and may be broken at these scored lines to provide the following doses: 75 mg, 50 mg and 25 mg.
100 mg treatment (take the whole tablet)
How to break your BENZNIDAZOLE 100 mg tablet:
• Hold the tablet between your thumbs and index fingers close to the scored line (See Figure 1).
• Apply enough pressure to break the tablet at the scored line (See Figure 2).
• Only use a tablet that has been broken at the scored line (See Figure 3).
•Do notbreak the BENZNIDAZOLE 100 mg tablet in any other way.
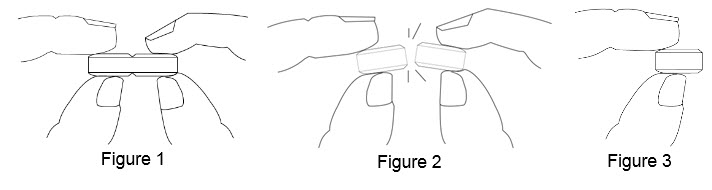
75 mg treatment(take one-half of the tablet and one-fourth of the tablet)
50 mg treatment(take one-half of the tablet)
25 mg treatment(take one-fourth of the tablet)
How should I store BENZNIDAZOLE?
• Store BENZNIDAZOLE at room temperature 20° to 25°C (68°to 77°F).
• Keep BENZNIDAZOLE in the bottle that it comes in and keep the bottle tightly closed.
• Keep BENZNIDAZOLE away from moisture.
Keep BENZNIDAZOLE and all medicines out of the reach of children.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by: Laboratori os Liconsa S.A., Guadalajara, Spain
Issued: August 2017
