Registrants1
Companies and organizations registered with the FDA for this drug approval, including their contact information and regulatory details.
791119022
Manufacturing Establishments1
FDA-registered manufacturing facilities and establishments involved in the production, packaging, or distribution of this drug product.
Preferred Pharmaceuticals, Inc.
Preferred Pharmaceuticals, Inc.
791119022
Products1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
PROCTOZONE-HC
Product Details
Drug Labeling Information
Complete FDA-approved labeling information including indications, dosage, warnings, contraindications, and other essential prescribing details.
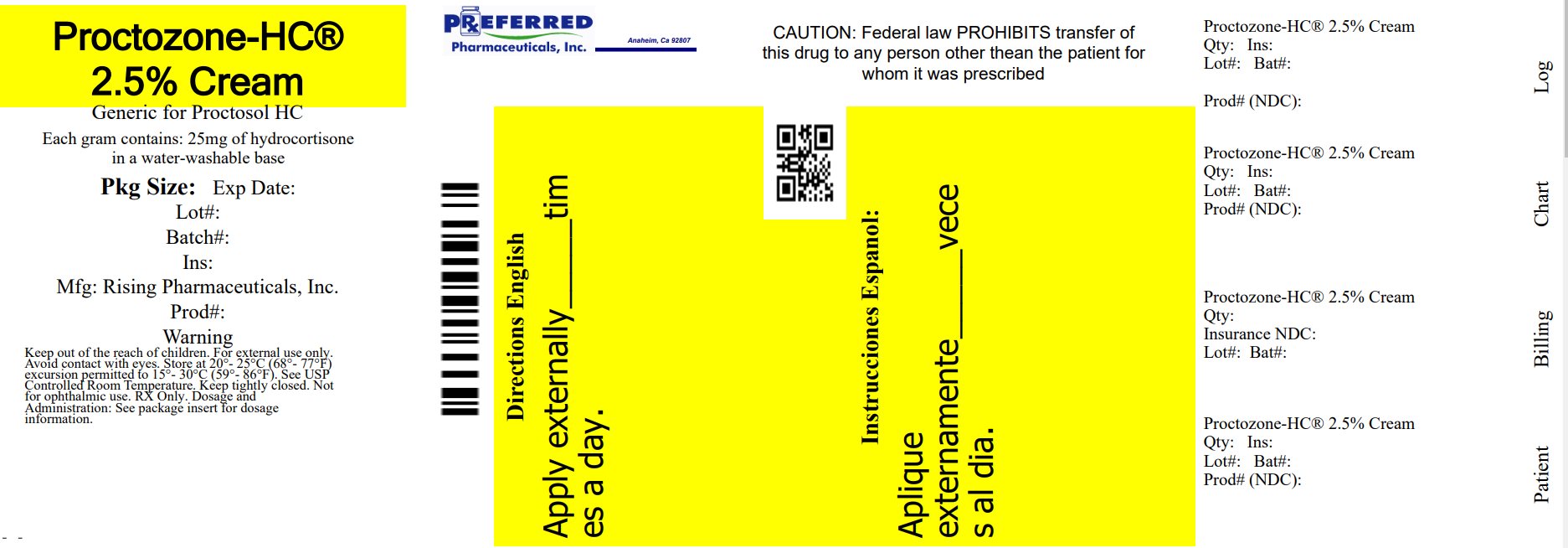
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
———Principal Display Panel———
Proctozone-HC**®**** 2.5%**
** (Hydrocortisone Cream, USP 2.5%)**
Rx only
** For External Use Only**
** Not for Ophthalmic Use**
** 30 g (1.1 oz) Tube**
Relabeled By: Preferred Pharmaceuticals Inc.
DESCRIPTION SECTION
DESCRIPTION
Each gram of Proctozone-HC® (Hydrocortisone Cream, USP 2.5%) contains 25 mg of hydrocortisone in a water-washable base of purified water, propylene glycol, glyceryl monostearate SE, cholesterol, isopropyl myristate, polysorbate 60, cetyl alcohol, sorbitan monostearate, polyoxyl 40 stearate, sorbic acid, methylparaben, and propylparaben.
Chemically, hydrocortisone is [Pregn-4-ene-3, 20-dione, 11, 17, 21-trihydroxy-, (11β)-] with the molecular formula (C21H30O5) and is represented by the following structural formula:

Its molecular weight is 362.46 and its CAS Registry Number is 50-23-7. The topical corticosteroids, including hydrocortisone, constitute a class of primarily synthetic steroids used as anti-inflammatory and antipruritic agents.
HOW SUPPLIED SECTION
HOW SUPPLIED
PROCTOZONE-HC® 2.5%
(Hydrocortisone Cream, USP 2.5%)
Tubes containing 30 g (1.1 oz)
NDC 68788-7091-3
Storage
Keep tightly closed. Store at 20° – 25° C (68° – 77° F) excursion permitted to
15° – 30° C
(59° – 86° F) [See USP Controlled Room Temperature].
Keep out of the reach of children.
|
Distributed by: |
** Manufactured by:** |
|
** Rising Pharmaceuticals, Inc.** |
** Lyne Laboratories, Inc.** |
|
** East Brunswick, NJ 08816** |
** Brockton, MA 02301** |
R4-02/23 PIR32430-01
