Permethrin
Permethrin Cream, 5%*
552731d1-69db-03bc-e054-00144ff88e88
HUMAN PRESCRIPTION DRUG LABEL
Feb 12, 2021
NuCare Pharmaceuticals,Inc.
DUNS: 010632300
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Permethrin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (13)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Principal Display Panel

INDICATIONS & USAGE SECTION
INDICATIONS AND USAGE
Permethrin Cream, 5% is indicated for the treatment of infestation with Sarcoptes scabiei (scabies).
CONTRAINDICATIONS SECTION
CONTRAINDICATIONS
Permethrin Cream, 5% is contraindicated in patients with known hypersensitivity to any of its components, to any synthetic pyrethroid or pyrethrin.
ADVERSE REACTIONS SECTION
ADVERSE REACTIONS
In clinical trials, generally mild and transient burning and stinging followed application with permethrin cream, 5% in 10% of patients and was associated with the severity of infestation. Pruritus was reported in 7% of patients at various times post-application. Erythema, numbness, tingling, and rash were reported in 1 to 2% or less of patients (see PRECAUTIONS-General). Other adverse events reported since marketing permethrin cream, 5% include: headache, fever, dizziness, abdominal pain, diarrhea and nausea and/or vomiting. Although extremely uncommon and not expected when used as directed (see DOSAGE AND ADMINISTRATION), rare occurrences of seizure have been reported. None have been medically confirmed as associated with Permethrin Cream, 5% treatment.
SPL UNCLASSIFIED SECTION
*w/w
Rx Only
DESCRIPTION SECTION
DESCRIPTION
Permethrin Cream, 5% is a topical scabicidal agent for the treatment of infestation with Sarcoptes scabiei (scabies). It is available in an off-white, vanishing cream base. Permethrin Cream, 5% is for topical use only.
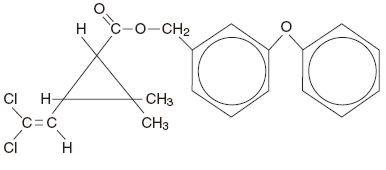
Chemical Name – The permethrin used is an approximate 1:3 mixture of the cis and trans isomers of the pyrethroid 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylic acid, (3-phenoxyphenyl) methyl ester. Permethrin has a molecular formula of C 21H 20Cl 2O 3 and a molecular weight of 391.29. It is a yellow to light orange- brown, low melting solid or viscous liquid.
Active Ingredient – Each gram contains permethrin 50 mg (5%).
Inactive Ingredients – Butylated hydroxytoluene, carbomer homopolymer type B, fractionated coconut oil, glycerin, glyceryl monostearate, isopropyl myristate, lanolin alcohols, mineral oil, polyoxyethylene cetyl ethers, purified water, and sodium hydroxide. Formaldehyde 1 mg (0.1%) is added as a preservative.
CLINICAL PHARMACOLOGY SECTION
CLINICAL PHARMACOLOGY
Permethrin, a pyrethroid, is active against a broad range of pests including lice, ticks, fleas, mites, and other arthropods. It acts on the nerve cell membrane to disrupt the sodium channel current by which the polarization of the membrane is regulated. Delayed repolarization and paralysis of the pests are the consequences of this disturbance.
Permethrin is rapidly metabolized by ester hydrolysis to inactive metabolites which are excreted primarily in the urine. Although the amount of permethrin absorbed after a single application of the 5% cream has not been determined precisely, data from studies with 14C-labeled permethrin and absorption studies of the cream applied to patients with moderate to severe scabies indicate it is 2% or less of the amount applied.
WARNINGS SECTION
WARNINGS
If hypersensitivity to Permethrin Cream, 5% occurs, discontinue use.
PRECAUTIONS SECTION
PRECAUTIONS
General -
Scabies infestation is often accompanied by pruritus, edema, and erythema. Treatment with Permethrin Cream, 5% may temporarily exacerbate these conditions.
Information for Patients -
Patients with scabies should be advised that itching, mild burning and/or stinging may occur after application of Permethrin Cream, 5%. In clinical trials, approximately 75% of patients treated with permethrin cream, 5% who continued to manifest pruritus at 2 weeks had cessation by 4 weeks. If irritation persists, they should consult their physician. Permethrin Cream, 5% may be very mildly irritating to the eyes. Patients should be advised to avoid contact with eyes during application and to flush with water immediately if Permethrin Cream, 5% gets in the eyes.
Carcinogenesis, Mutagenesis, Impairment of Fertility -
Six carcinogenicity bioassays were evaluated with permethrin, three each in rats and mice. No tumorigenicity was seen in the rat studies. However, species-specific increases in pulmonary adenomas, a common benign tumor of mice of high spontaneous background incidence, were seen in the three mouse studies. In one of these studies there was an increased incidence of pulmonary alveolar-cell carcinomas and benign liver adenomas only in female mice when permethrin was given in their food at a concentration of 5000 ppm. Mutagenicity assays, which give useful correlative data for interpreting results from carcinogenicity bioassays in rodents, were negative. Permethrin showed no evidence of mutagenic potential in a battery of in vitro and in vivo genetic toxicity studies.
Permethrin did not have any adverse effect on reproductive function at a dose of 180 mg/kg/day orally in a three-generation rat study.
Pregnancy: Teratogenic Effects: Pregnancy Category B -
Reproduction studies have been performed in mice, rats, and rabbits (200 to 400 mg/kg/day orally) and have revealed no evidence of impaired fertility or harm to the fetus due to permethrin. There are, however, no adequate and well- controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers -
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the evidence for tumorigenic potential of permethrin in animal studies, consideration should be given to discontinuing nursing temporarily or withholding the drug while the mother is nursing.
Pediatric Use -
Permethrin Cream, 5% is safe and effective in pediatric patients two months of age and older. Safety and effectiveness in infants less than two months of age have not been established.
Geriatric Use -
Clinical studies of permethrin cream, 5% did not identify sufficient numbers of subjects aged 65 and over to allow a definitive statement regarding whether elderly subjects respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. This drug is known to be substantially excreted by the kidney. However, since topical permethrin is metabolized in the liver and excreted in the urine as inactive metabolites, there does not appear to be an increased risk of toxic reactions in patients with impaired renal function when used as labeled.
OVERDOSAGE SECTION
OVERDOSAGE
No instance of accidental ingestion of Permethrin Cream, 5% has been reported. If ingested, gastric lavage and general supportive measures should be employed. Excessive topical use (seeDOSAGE AND ADMINISTRATION) may result in increased irritation and erythema.
DOSAGE & ADMINISTRATION SECTION
DOSAGE AND ADMINISTRATION
Adults and children –
Thoroughly massage Permethrin Cream, 5% into the skin from the head to the soles of the feet. Scabies rarely infests the scalp of adults, although the hairline, neck, temple, and forehead may be infested in infants and geriatric patients. Usually 30 grams is sufficient for an average adult. The cream should be removed by washing (shower or bath) after 8 to 14 hours. Infants should be treated on the scalp, temple, and forehead.ONE APPLICATION IS GENERALLY CURATIVE.
HOW SUPPLIED SECTION
HOW SUPPLIED
Permethrin Cream, 5% (w/w) is available as follows:
NDC 68071-3316 Box of 60 g
STORAGE AND HANDLING SECTION
STORAGE
Store at 20-25°C (68-77°F) [see USP Controlled Room Temperature].
Manufactured by Perrigo
Bronx, NY 10457
Distributed By
Perrigo®
Allegan, MI 49010 • www.perrigo.com
Rev 08-15
: 1T600 RC JX1
