Ondansetron
These highlights do not include all the information needed to use ONDANSETRON TABLETS and ONDANSETRON ORALLY DISINTEGRATING TABLETS safely and effectively. See full prescribing information for ONDANSETRON TABLETS and ONDANSETRON ORALLY DISINTEGRATING TABLETS. Initial U.S. Approval: 1991
c0d827d0-333f-4187-ac75-9dc7d34b6169
HUMAN PRESCRIPTION DRUG LABEL
Aug 20, 2025
REMEDYREPACK INC.
DUNS: 829572556
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Ondansetron
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
DRUG: Ondansetron
GENERIC: Ondansetron
DOSAGE: TABLET, ORALLY DISINTEGRATING
ADMINSTRATION: ORAL
NDC: 70518-3512-0
NDC: 70518-3512-1
NDC: 70518-3512-2
NDC: 70518-3512-3
NDC: 70518-3512-4
NDC: 70518-3512-5
NDC: 70518-3512-6
NDC: 70518-3512-7
NDC: 70518-3512-8
COLOR: white
FLAVOR: STRAWBERRY
SHAPE: ROUND
SCORE: No score
SIZE: 7 mm
IMPRINT: G;4
PACKAGING: 30 in 1 BOX, UNIT-DOSE
PACKAGING: 6 in 1 BOX, UNIT-DOSE
PACKAGING: 10 in 1 BOX, UNIT-DOSE
PACKAGING: 20 in 1 BOX, UNIT-DOSE
PACKAGING: 12 in 1 BOX, UNIT DOSE
PACKAGING: 9 in 1 BOX, UNIT DOSE
PACKAGING: 21 in 1 BOX, UNIT DOSE
PACKAGING: 15 in 1 BOX, UNIT DOSE
PACKAGING: 3 in 1 BOX, UNIT DOSE
ACTIVE INGREDIENT(S):
- ONDANSETRON 4mg in 1
INACTIVE INGREDIENT(S):
- ASPARTAME
- SILICON DIOXIDE
- CROSPOVIDONE (120 .MU.M)
- MAGNESIUM STEARATE
- MANNITOL
- SODIUM STEARYL FUMARATE





ADVERSE REACTIONS SECTION
6. Adverse Reactions
RECENT MAJOR CHANGES SECTION
DESCRIPTION SECTION
11 DESCRIPTION
The active ingredient in Ondansetron Tablets, USP is ondansetron hydrochloride, USP as the dihydrate, the racemic form of ondansetron and a selective blocking agent of the serotonin 5-HT 3 receptor type. Chemically it is (±) 1, 2, 3, 9-tetrahydro-9-methyl-3-[(2-methyl-1H-imidazol-1-yl)methyl]-4H-carbazol-4-one, monohydrochloride, dihydrate. It has the following structural formula:
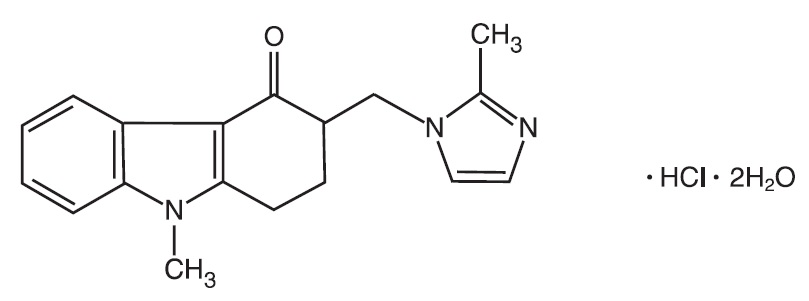
The empirical formula is C 18H 19N 3O•HCl•2H 2O, representing a molecular weight of 365.85 g/mol.
Ondansetron hydrochloride, USP (dihydrate) is a white to off-white powder that is sparingly soluble in water and in alcohol; soluble in methanol, slightly soluble in isopropyl alcohol, and in dichloromethane; very slightly soluble in acetone, in chloroform and in ethyl acetate.
The active ingredient in Ondansetron Orally Disintegrating Tablets, USP is ondansetron base, the racemic form of ondansetron, and a selective blocking agent of the serotonin 5-HT 3 receptor type. Chemically it is (±) 1, 2, 3, 9-tetrahydro-9-methyl-3-[(2-methyl-1H-imidazol-1-yl)methyl]-4H-carbazol-4-one. It has the following structural formula:
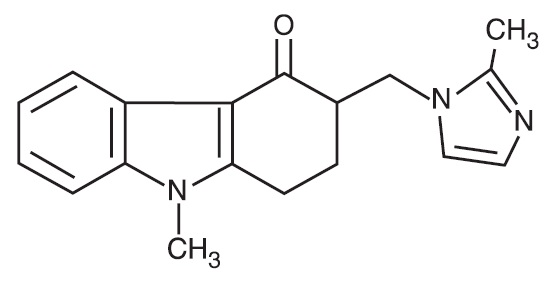
The empirical formula is C 18H 19N 3O representing a molecular weight of 293.4 g/mol.
Each 4-mg Ondansetron Tablet, USP for oral administration contains ondansetron hydrochloride, USP (dihydrate) equivalent to 4 mg of ondansetron. Each 8-mg Ondansetron Tablet, USP for oral administration contains ondansetron hydrochloride, USP (dihydrate) equivalent to 8 mg of ondansetron. Each tablet also contains the inactive ingredients colloidal silicon dioxide, hypromellose, iron oxide yellow (8 mg tablet only), lactose monohydrate, magnesium stearate, microcrystalline cellulose, pregelatinized starch, titanium dioxide and triacetin.
Each 4-mg Ondansetron Orally Disintegrating Tablet, USP for oral administration contains 4 mg ondansetron base. Each 8-mg Ondansetron Orally Disintegrating Tablet, USP for oral administration contains 8 mg ondansetron base. Each Ondansetron Orally Disintegrating Tablet, USP also contains the inactive ingredients aspartame, colloidal silicon dioxide, crospovidone, magnesium stearate, mannitol, sodium stearyl fumarate and strawberry flavor. Ondansetron Orally Disintegrating Tablets, USP are an orally administered formulation of ondansetron which rapidly disintegrates on the tongue and does not require water to aid dissolution or swallowing. This product disintegrates in approximately 60 seconds.
Ondansetron Orally Disintegrating Tablets, USP meet USP Disintegration Test 2.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Ondansetron Orally Disintegrating Tablets, USP
4 mg (as 4 mg ondansetron base) are white, circular, flat faced, uncoated tablets with ‘G’ engraved on one side and ‘4’ on the other side in:
NDC: 70518-3512-00
NDC: 70518-3512-01
NDC: 70518-3512-02
NDC: 70518-3512-03
NDC: 70518-3512-04
NDC: 70518-3512-05
NDC: 70518-3512-06
NDC: 70518-3512-07
NDC: 70518-3512-08
PACKAGING: 30 in 1 BOX, UNIT DOSE
PACKAGING: 6 in 1 BOX, UNIT DOSE
PACKAGING: 10 in 1 BOX, UNIT DOSE
PACKAGING: 20 in 1 BOX, UNIT DOSE
PACKAGING: 12 in 1 BOX, UNIT DOSE
PACKAGING: 9 in 1 BOX, UNIT DOSE
PACKAGING: 21 in 1 BOX, UNIT DOSE
PACKAGING: 15 in 1 BOX, UNIT DOSE
PACKAGING: 3 in 1 BOX, UNIT DOSE
Store at 20°C to 25°C (68°F to 77°F) [see USP Controlled Room Temperature].
Repackaged and Distributed By:
Remedy Repack, Inc.
625 Kolter Dr. Suite #4 Indiana, PA 1-724-465-8762
