Micafungin
These highlights do not include all the information needed to use MICAFUNGIN FOR INJECTION safely and effectively. See full prescribing information for MICAFUNGIN FOR INJECTION. MICAFUNGIN for injection, for intravenous use Initial U.S. Approval: 2005
29f17423-5787-47d0-a83b-bbdc0198fbfa
HUMAN PRESCRIPTION DRUG LABEL
Jun 24, 2023
Zydus Pharmaceuticals USA Inc.
DUNS: 156861945
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Micafungin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
Micafungin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
Drug Labeling Information
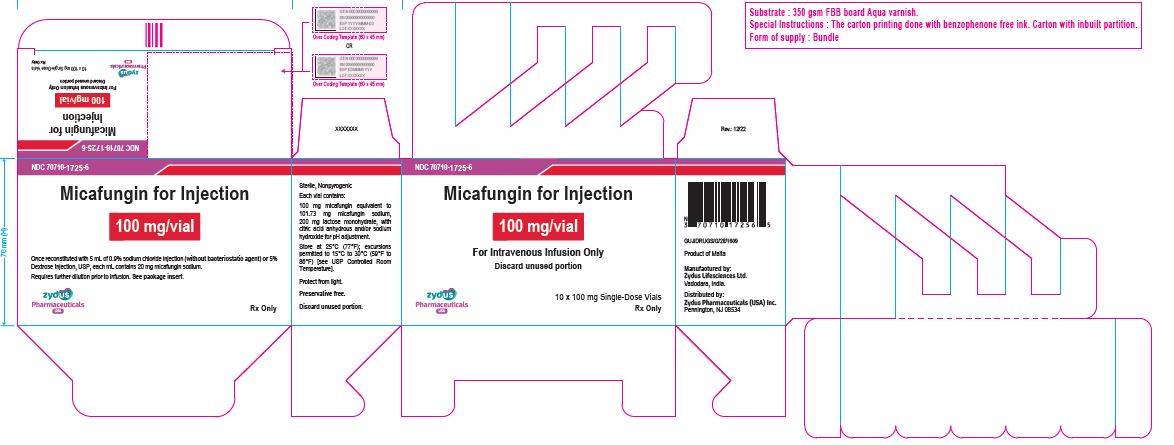
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 70710-1724-6
Micafungin for Injection
50 mg/vial
For Intravenous Infusion Only
10 x 50 mg Single-Dose Vials
Rx only

NDC 70710-1725-6
Micafungin for Injection
100 mg/vial
For Intravenous Infusion Only
10 x 100 mg Single-Dose Vials
Rx only
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
Micafungin for injection is indicated for:
- Treatment of Candidemia, Acute Disseminated Candidiasis, CandidaPeritonitis and Abscesses in adult and pediatric patients 4 months of age and older [see Clinical Studies (14.1) and Use in Specific Populations (8.4)].
- Treatment of Candidemia, Acute Disseminated Candidiasis, Candida Peritonitis and Abscesseswithoutmeningoencephalitis and/or ocular dissemination in pediatric patients younger than 4 months of age [see Use in Specific Populations (8.4)].
- Treatment of Esophageal Candidiasis in adult and pediatric patients 4 months of age and older [see Clinical Studies (14.2)].
- Prophylaxis of CandidaInfections in adult and pediatric patients 4 months of age and older undergoing hematopoietic stem cell transplantation [see Clinical Studies (14.3)].
Limitations of Use
- The safety and effectiveness of Micafungin havenotbeen established for the treatment of candidemiawithmeningoencephalitis and/or ocular dissemination in pediatric patients younger than 4 months of age as a higher dose may be needed [see Use in Specific Populations (8.4)].
- Micafungin has not been adequately studied in patients with endocarditis, osteomyelitis and meningoencephalitis due to Candida.
- The efficacy of micafungin against infections caused by fungi other than Candidahas not been established.
Micafungin for injection is an echinocandin indicated in adult and pediatric patients for (1):
- Treatment of Candidemia, Acute Disseminated Candidiasis, CandidaPeritonitis and Abscesses in adult and pediatric patients 4 months of age and older.
- Treatment of Candidemia, Acute Disseminated Candidiasis, Candida Peritonitis and Abscesseswithoutmeningoencephalitis and/or ocular dissemination in pediatric patients younger than 4 months of age.
- Treatment of Esophageal Candidiasis in adult and pediatric patients 4 months of age and older.
- Prophylaxis of CandidaInfections in adult and pediatric patients 4 months of age and older undergoing Hematopoietic Stem Cell Transplantation (HSCT).
Limitations of Use
- The safety and effectiveness of Micafungin have not been established for the treatment of candidemiawithmeningoencephalitis and/or ocular dissemination in pediatric patients younger than 4 months of age as a higher dose may be needed. (1, 2.3, 8.4)
- Micafungin has not been adequately studied in patients with endocarditis, osteomyelitis or meningoencephalitis due to Candida.(1)
- The efficacy of micafungin against infections caused by fungi other than Candidahas not been established. (1)
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Isolated cases of serious hypersensitivity (anaphylaxis and anaphylactoid) reactions (including shock) have been reported in patients receiving micafungin. If these reactions occur, micafungin infusion should be discontinued and appropriate treatment administered.
5.2 Hematological Effects
Acute intravascular hemolysis and hemoglobinuria was seen in a healthy volunteer during infusion of micafungin (200 mg) and oral prednisolone (20 mg). Cases of significant hemolysis and hemolytic anemia have also been reported in patients treated with micafungin. Patients who develop clinical or laboratory evidence of hemolysis or hemolytic anemia during micafungin therapy should be monitored closely for evidence of worsening of these conditions and evaluated for the risk/benefit of continuing micafungin therapy.
5.3 Hepatic Effects
Laboratory abnormalities in liver function tests have been seen in healthy volunteers and patients treated with micafungin. In some patients with serious underlying conditions who were receiving micafungin along with multiple concomitant medications, clinical hepatic abnormalities have occurred and isolated cases of significant hepatic impairment, hepatitis and hepatic failure have been reported. Patients who develop abnormal liver function tests during micafungin therapy should be monitored for evidence of worsening hepatic function and evaluated for the risk/benefit of continuing micafungin therapy.
5.4 Renal Effects
Elevations in BUN and creatinine, and isolated cases of significant renal impairment or acute renal failure have been reported in patients who received micafungin. In fluconazole-controlled trials, the incidence of drug-related renal adverse reactions was 0.4% for micafungin-treated patients and 0.5% for fluconazole-treated patients. Patients who develop abnormal renal function tests during micafungin therapy should be monitored for evidence of worsening renal function.
5.5 Infusion and Injection Site Reactions
Possible histamine-mediated symptoms have been reported with micafungin, including rash, pruritus, facial swelling and vasodilatation. Slow the infusion rate if infusion reaction occurs [see Dosage and Administration (2.3)].
Injection site reactions, including phlebitis and thrombophlebitis have been reported, at micafungin doses of 50 mg/day to 150 mg/day. These reactions tended to occur more often in patients receiving micafungin via peripheral intravenous administration [see Dosage and Administration (2.3) and Adverse Reactions (6.1)].
- Hypersensitivity Reactions: Anaphylaxis and anaphylactoid reactions (including shock) have been observed. Discontinue micafungin and administer appropriate treatment. (5.1)
- Hematological Effects: Isolated cases of acute intravascular hemolysis, hemolytic anemia and hemoglobinuria have been reported. Monitor rate of hemolysis. Discontinue if severe. (5.2)
- Hepatic Effects: Abnormalities in liver tests; isolated cases of hepatic impairment, hepatitis and hepatic failure have been observed. Monitor hepatic function. Discontinue if severe dysfunction occurs. (5.3)
- Renal Effects: Elevations in BUN and creatinine; isolated cases of renal impairment or acute renal failure have been reported. Monitor renal function. (5.4)
- Infusion and Injection Site Reactions can occur including rash, pruritus, facial swelling and vasodilatation. Monitor infusion closely, slow infusion rate if necessary. (2.5, 5.5)
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on Micafungin
CYP3A4, CYP2C9 and CYP2C19 Inhibitors
Co-administration of micafungin with cyclosporine, itraconazole, voriconazole and fluconazole did not alter the pharmacokinetics of micafungin.
CYP2C19 and CYP3A4 Inducer
Co-administration of micafungin with rifampin and ritonavir did not alter the pharmacokinetics of micafungin.
Co-administration of Micafungin with Other Drugs
Co-administration of micafungin with mycophenolate mofetil (MMF), amphotericin B, tacrolimus, prednisolone, sirolimus and nifedipine did not alter the pharmacokinetics of micafungin.
7.2 Effect of Micafungin on Other Drugs
CYP3A4 Substrates
There was no effect of single or multiple doses of micafungin on cyclosporine, tacrolimus, prednisolone, voriconazole and fluconazole pharmacokinetics.
Sirolimus AUC was increased by 21% with no effect on Cmax in the presence of steady-state micafungin compared with sirolimus alone. Nifedipine AUC and Cmax were increased by 18% and 42%, respectively, in the presence of steady-state micafungin compared with nifedipine alone. Itraconazole AUC and Cmax were increased by 22% and 11%, respectively. Patients receiving sirolimus, nifedipine and itraconazole in combination with micafungin should be monitored for sirolimus, nifedipine and itraconazole toxicity and the sirolimus, nifedipine and itraconazole dosage should be reduced if necessary.
UDP-Glycosyltransferase Substrate
Co-administration of mycophenolate mofetil (MMF) with micafungin did not alter the pharmacokinetics of MMF.
Monitor for sirolimus, itraconazole or nifedipine toxicity and dosage of sirolimus, itraconazole or nifedipine should be reduced, if necessary. (7)
OVERDOSAGE SECTION
10 OVERDOSAGE
Micafungin is highly protein bound and, therefore, is not dialyzable. No cases of micafungin overdosage have been reported. Repeated daily doses up to 8 mg/kg (maximum total dose of 896 mg) in adult patients, up to 6 mg/kg in pediatric patients 4 months of age and older, and up to 15 mg/kg in pediatric patients younger than 4 months of age have been administered in clinical trials with no reported dose-limiting toxicity [see Adverse Reactions (6.1) and Use in Specific Populations (8.4)].
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Hepatic carcinomas and adenomas were observed in a 6 month intravenous toxicology study with an 18 month recovery period of micafungin sodium in rats designed to assess the reversibility of hepatocellular lesions.
Rats administered micafungin sodium for 3 months at 32 mg/kg/day (corresponding to 8 times the highest recommended human dose [150 mg/day], based on AUC comparisons), exhibited colored patches/zones, multinucleated hepatocytes and altered hepatocellular foci after 1 month or 3 month recovery periods and adenomas were observed after a 21 month recovery period. Rats administered micafungin sodium at the same dose for 6 months exhibited adenomas after a 12 month recovery period; after an 18 month recovery period, an increased incidence of adenomas was observed and, additionally, carcinomas were detected. A lower dose of micafungin sodium (equivalent to 5 times the human AUC) in the 6 month rat study resulted in a lower incidence of adenomas and carcinomas following 18 months recovery. The duration of micafungin dosing in these rat studies (3 months or 6 months) exceeds the usual duration of micafungin dosing in patients, which is typically less than 1 month for treatment of esophageal candidiasis, but dosing may exceed 1 month for Candida prophylaxis.
Although the increase in carcinomas in the 6 month rat study did not reach statistical significance, the persistence of altered hepatocellular foci subsequent to micafungin dosing and the presence of adenomas and carcinomas in the recovery periods suggest a causal relationship between micafungin sodium, altered hepatocellular foci and hepatic neoplasms. Whole-life carcinogenicity studies of micafungin in animals have not been conducted and it is not known whether the hepatic neoplasms observed in treated rats also occur in other species or if there is a dose threshold for this effect.
Micafungin sodium was not mutagenic or clastogenic when evaluated in a standard battery of in vitro and in vivo tests (i.e., bacterial reversion - S. typhimurium, E. coli; chromosomal aberration; intravenous mouse micronucleus).
Male rats treated intravenously with micafungin sodium for 9 weeks showed vacuolation of the epididymal ductal epithelial cells at or above 10 mg/kg (about 0.6 times the recommended clinical dose for esophageal candidiasis, based on body surface area comparisons). Higher doses (about twice the recommended clinical dose, based on body surface area comparisons) resulted in higher epididymis weights and reduced numbers of sperm cells. In a 39 week intravenous study in dogs, seminiferous tubular atrophy and decreased sperm in the epididymis were observed at 10 mg/kg and 32 mg/kg, doses equal to about 2 times and 7 times the recommended clinical dose, based on body surface area comparisons. There was no impairment of fertility in animal studies with micafungin sodium.
13.2 Animal Toxicology and/or Pharmacology
High doses of micafungin sodium (5 times to 8 times the highest recommended human dose, based on AUC comparisons) have been associated with irreversible changes to the liver when administered for 3 months or 6 months and these changes may be indicative of pre-malignant processes [see Nonclinical Toxicology (13.1)].
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Micafungin for injection is supplied as a sterile, white color lyophilized powder or cake in an amber glass vial for reconstitution for intravenous infusion and are available in the following packaging configurations:
|
** Strength** |
** Pack Size** |
** Carton NDC** |
|
50 mg/vial |
Carton of 10 Single-dose vials sealed with blue flip-off cap |
70710-1724-6 |
|
100 mg/vial |
Carton of 10 Single-dose vials sealed with red flip-off cap |
70710-1725-6 |
Storage
Unopened vials of lyophilized material must be stored at room temperature, 25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
Store the reconstituted product at 25°C (77°F) [see Dosage and Administration (2.4)].
Store the diluted solution at 25°C (77°F) [see Dosage and Administration (2.4)].
Protect from light.
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Dosage for Adults
The recommended dosage for adult patients based on indications are shown in Table 1.
Table 1 Micafungin Dosage in Adult Patients
| |
|
‡ In patients treated successfully for esophageal candidiasis, the mean duration of treatment was 15 days (range 10 days to 30 days). | |
|
§ In hematopoietic stem cell transplant (HSCT) recipients who experienced success of prophylactic therapy, the mean duration of prophylaxis was 19 days (range 6 days to 51 days). | |
|
** Indication** |
** Recommended Reconstituted Dose Once Daily** |
|
Treatment of Candidemia, Acute Disseminated Candidiasis, Candida Peritonitis and Abscesses* |
** 100 mg** |
|
Treatment of Esophageal Candidiasis‡ |
** 150 mg** |
|
Prophylaxis of Candida Infections in HSCT Recipients§ |
** 50 mg** |
2.2 Dosage for Pediatric Patients 4 Months and Older
The recommended dosage for pediatric patients 4 months of age and older based on indication and weight are shown in Table 2.
Table 2 Micafungin Dosage in Pediatric Patients (4 Months of Age and Older)|
** Indication** |
** Dosage for Pediatric Patients 4 Months of Age and Older** | |
|
** 30 kg or less** |
** Greater than 30 kg** | |
|
Treatment of Candidemia, Acute Disseminated Candidiasis, Candida Peritonitis and Abscesses |
2 mg/kg once daily (maximum daily dose 100 mg) | |
|
Treatment of Esophageal Candidiasis |
3 mg/kg once daily |
2.5 mg/kg once daily (maximum daily dose 150 mg) |
|
Prophylaxis of Candida Infections in HSCT Recipients |
1 mg/kg once daily (maximum daily dose 50 mg) |
2.3 Dosage for Pediatric Patients Younger than 4 Months of Age
Treatment of Candidemia, Acute Disseminated Candidiasis, Candida Peritonitis and Abscesseswithoutmeningoencephalitis and/or ocular dissemination
The recommended dosage is 4 mg/kg once daily.
The safety and effectiveness of MYCAMINE havenotbeen established for the treatment of candidemiawithmeningoencephalitis and/or ocular dissemination in pediatric patients younger than 4 months of age as a higher dose may be needed [see Use in Specific Populations (8.4), Clinical Pharmacology (12.3) and Microbiology (12.4)].
2.4 Directions for Reconstitution, Dilution and Preparation
Do not mix or co-infuse micafungin with other medications. Micafungin has been shown to precipitate when mixed directly with a number of other commonly used medications. Please read this entire section carefully before beginning reconstitution.
Reconstitution
Reconstitute micafungin vials by aseptically adding 5 mL of one of the following compatible solutions:
- 0.9% Sodium Chloride Injection, USP (without a bacteriostatic agent)
- 5% Dextrose Injection, USP
To minimize excessive foaming, gently dissolve the micafungin powder by swirling the vial. Do not vigorously shake the vial. Visually inspect the vial for particulate matter.
Micafungin for injection 50 mg vial: after reconstitution each mL contains 10 mg of micafungin.
Micafungin for injection 100 mg vial: after reconstitution each mL contains 20 mg of micafungin.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if there is any evidence of precipitation or foreign matter. Aseptic technique must be strictly observed in all handling since no preservative or bacteriostatic agent is present in micafungin for injection or in the materials specified for reconstitution and dilution.
The reconstituted product should be protected from light and may be stored in the original vial for up to 24 hours at room temperature, 25°C (77°F).
Dilution and Preparation
The diluted solution should be protected from light. It is not necessary to cover the infusion drip chamber or the tubing.
Adult Patients:
- Add the appropriate volume of reconstituted micafungin into 100 mL of 0.9% Sodium Chloride Injection, USP or 100 mL of 5% Dextrose Injection, USP.
- Appropriately label the bag.
Pediatric Patients
1. Calculate the total micafungin dose in milligrams (mg) by multiplying the recommended pediatric dose (mg/kg) for a given indication [see Table 2] and the weight of the patient in kilograms (kg).
2. To calculate the volume (mL) of drug needed, divide the calculated dose (mg) from step 1 by the final concentration of the selected reconstituted vial(s) (either 10 mg/mL for the 50 mg vialor 20 mg/mL for the 100 mg vial), see example below:
Using 50 mg vials:
Divide the calculated mg dose (from step 1) by 10 mg/mL to determine the volume (mL) needed.
OR
Using 100 mg vials:
Divide the calculated mg dose (from step 1) by 20 mg/mL to determine the volume (mL) needed.
3. Withdraw the calculated volume (mL) of drug needed from the selected concentration and size of reconstituted micafungin vial(s) used in Step 2 (ensure the selected concentration and vial size used to calculate the dose is also used to prepare the infusion).
4. Add the withdrawn volume of drug (step 3) to a 0.9% Sodium Chloride Injection, USP or 5% Dextrose Injection, USP intravenous infusion bag or syringe. Ensure that the final concentration of the solution is between 0.5 mg/mL to 4 mg/mL.
To decrease the risk of infusion reactions, concentrations above 1.5 mg/mL should be administered via central catheter [see Warnings and Precautions (5.5)].
5. Appropriately label the infusion bag or syringe. For concentrations above 1.5 mg/mL, if required, label to specifically warn to administer the solution via central catheter.
The diluted infusion bag should be protected from light and may be stored for up to 24 hours at room temperature, 25°C (77°F).
Micafungin for injection is preservative-free. Discard partially used vials.
2.5 Infusion Volume and Duration
Administer micafungin by intravenous infusion only. Infuse over one hour. More rapid infusions may result in more frequent histamine-mediated reactions [see Warnings and Precautions (5.5)].
Flush an existing intravenous line with 0.9% Sodium Chloride Injection, USP, prior to infusion of Micafungin.
Pediatric Patients
Micafungin should be infused over one hour. To decrease the risk of infusion reactions, concentrations above 1.5 mg/mL should be administered via central catheter [see Warnings and Precautions (5.5)].
Recommended Dosage Administered by Indication, Weight and Age (2.1, 2.2,2.3,8.4)
|
** Adult** |
** Pediatric Patients 4 Months and Older 30 kg or less** |
** Pediatric Patients 4 Months and Older greater than 30 kg** |
** Pediatric Patients Younger than 4 Months of Age** |
|
** Treatment of Candidemia, Acute Disseminated Candidiasis,Candida Peritonitis and Abscesses** | |||
|
100 mg daily |
2 mg/kg/day (maximum 100 mg daily) |
See below | |
|
** Treatment of Candidemia, Acute Disseminated Candidiasis,Candida
Peritonitis and Abscesseswithout Meningoencephalitis and/or Ocular** | |||
|
See above |
See above |
4 mg/kg/day | |
|
** Treatment of Esophageal Candidiasis** | |||
|
150 mg daily |
3 mg/kg/day |
2.5 mg/kg/day (maximum 150 mg daily) |
Not approved |
|
** Prophylaxis ofCandida Infections in HSCT Recipients** | |||
|
50 mg daily |
1 mg/kg/day (maximum 50 mg daily) |
Not approved |
- Infuse over 1 hour. (2.5)
- See Full Prescribing Information for intravenous (IV) preparation and administration instructions. (2)
