Registrants1
Companies and organizations registered with the FDA for this drug approval, including their contact information and regulatory details.
171714327
Manufacturing Establishments1
FDA-registered manufacturing facilities and establishments involved in the production, packaging, or distribution of this drug product.
Bryant Ranch Prepack
Bryant Ranch Prepack
171714327
Products1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Levalbuterol
Product Details
Drug Labeling Information
Complete FDA-approved labeling information including indications, dosage, warnings, contraindications, and other essential prescribing details.
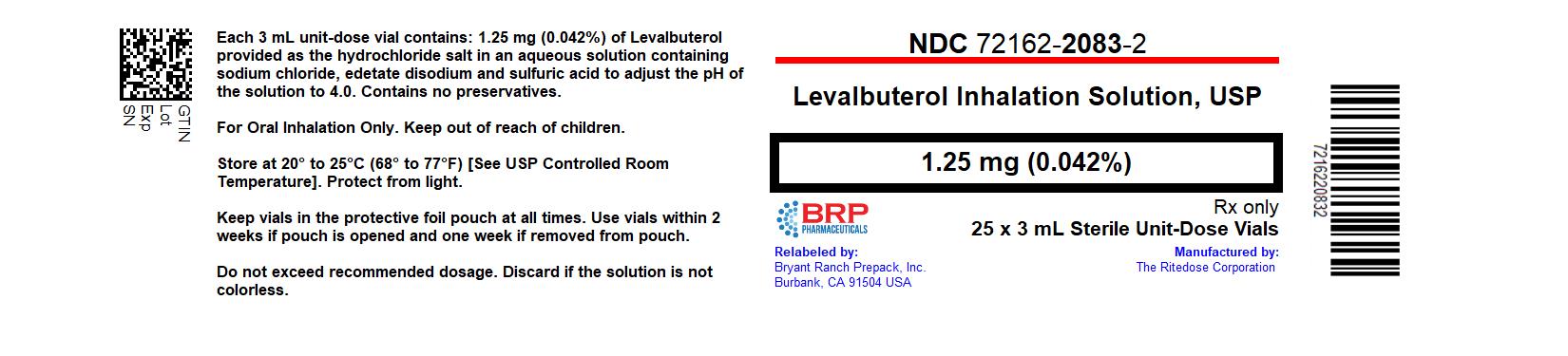
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Levalbuterol HCl 1.25 mg Solution #25