Aerowipe
Aerowipe Antiseptic Wipe
Approved
Approval ID
34ca12c7-9281-263c-e054-00144ff8d46c
Product Type
HUMAN OTC DRUG LABEL
Effective Date
Aug 1, 2025
Manufacturers
FDA
Aero Healthcare
DUNS: 008186174
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Anstiseptic Wipe
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
NDC Product Code55305-118
Application NumberM003
Product Classification
M
Marketing Category
C200263
G
Generic Name
Anstiseptic Wipe
Product Specifications
Route of AdministrationTOPICAL
Effective DateAugust 1, 2025
FDA Product Classification
INGREDIENTS (2)
WATERInactive
Code: 059QF0KO0R
Classification: IACT
BENZALKONIUM CHLORIDEActive
Quantity: 0.1 g in 100 g
Code: F5UM2KM3W7
Classification: ACTIB
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
LOINC: 51945-4Updated: 1/1/2016
Package Label
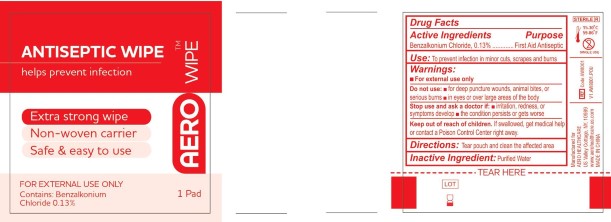
INDICATIONS & USAGE SECTION
LOINC: 34067-9Updated: 1/1/2016
USE
To prevent infection in minor cuts, scrapes and burns
OTC - STOP USE SECTION
LOINC: 50566-9Updated: 1/1/2016
Stop use and ask a doctor if:
- Irritation, redness, or symptoms develop
- the condition persists or gets worse
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
LOINC: 50565-1Updated: 1/1/2016
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
DOSAGE & ADMINISTRATION SECTION
LOINC: 34068-7Updated: 1/1/2016
Directions:
Tear pouch and clean the affected area.
INACTIVE INGREDIENT SECTION
LOINC: 51727-6Updated: 1/8/2016
Inactive Ingredient
Purified Water
OTC - ACTIVE INGREDIENT SECTION
LOINC: 55106-9Updated: 1/1/2016
Active Ingredient
Benzalkonium Chloride, 0.13%
OTC - PURPOSE SECTION
LOINC: 55105-1Updated: 1/1/2016
Purpose
First Aid Antiseptic
WARNINGS SECTION
LOINC: 34071-1Updated: 1/1/2016
Warnings:
For external use only
OTC - DO NOT USE SECTION
LOINC: 50570-1Updated: 1/1/2016
Do not use:
- For deep puncture wounds, animal bites, or serious burns
- In eyes or over large area of the body
