EyeOne Cleaner
37311080-b269-5ba2-e063-6394a90a2c6c
HUMAN OTC DRUG LABEL
Jun 10, 2025
IDO PHARM
DUNS: 694853523
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Hyaluronic Acid, Tocopheryl Acetate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
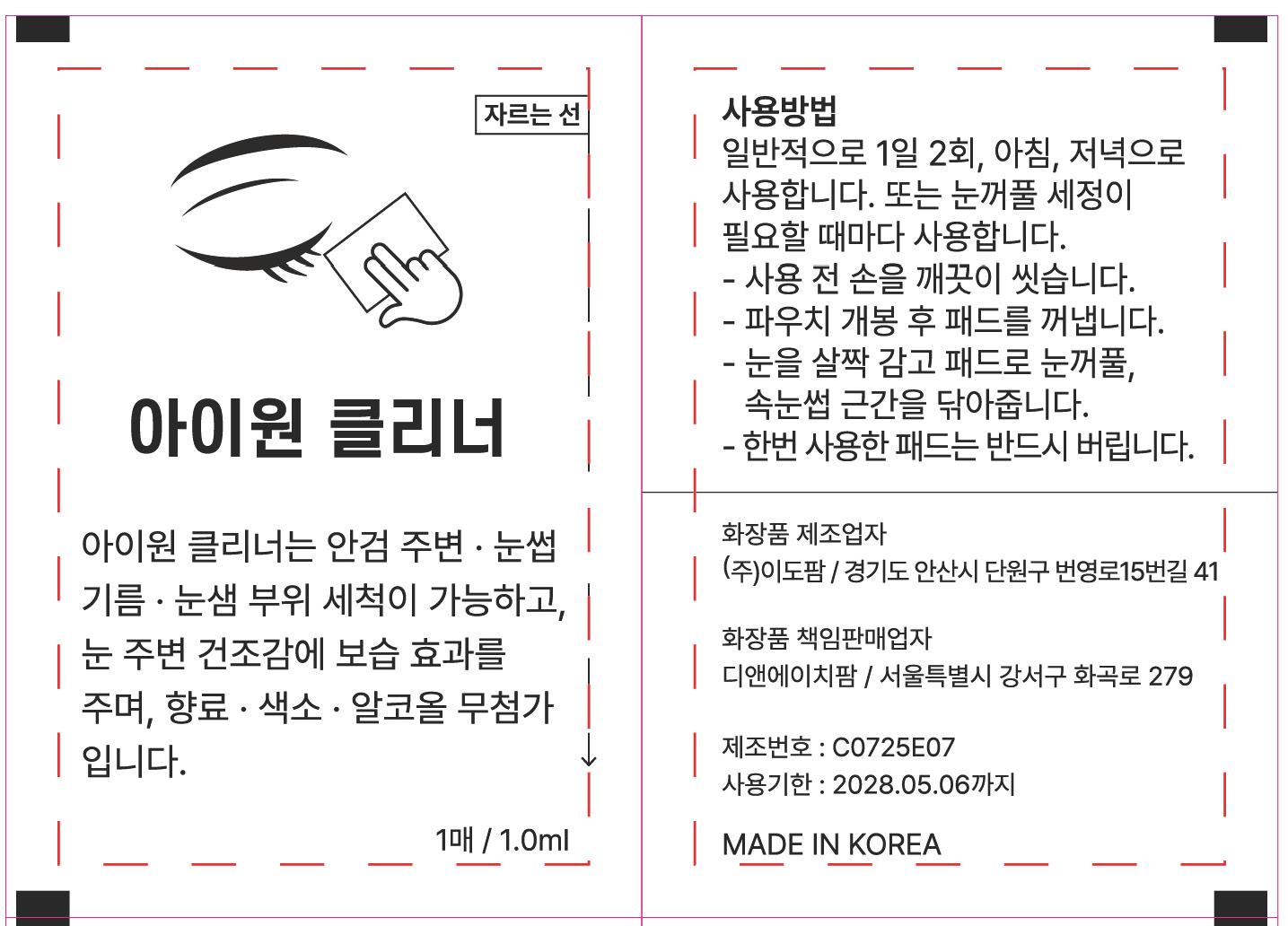

INDICATIONS & USAGE SECTION
Use it whenever you need to clean your eyelids
DOSAGE & ADMINISTRATION SECTION
It is generally used twice a day, in the morning and evening.
Or use it whenever you need to clean your eyelids.
- Wash your hands thoroughly before use.
- Remove the pad after opening the packet.
- Close your eyes and wipe the eyelids and the base of your eyelashes with
cloth.
- Once used, throw away cloth without fail.
OTC - ACTIVE INGREDIENT SECTION
Hyaluronic acid, Tocopheryl Acetate
OTC - PURPOSE SECTION
It cleans the area of eyebrow oil and glands and moisturizes the dryness around the eyes
WARNINGS SECTION
1. Consult a specialist if you have abnormal symptoms or side effects such as
red spots, swelling, or itching due to direct sunlight during or after using
this product.
2. Please refrain from using the wound area
3. Precautions for Storage and Handling
A. Please keep it out of the reach of children.
B. Please store it in a cool place.
4. Wash it immediately when it gets into your eyes.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
keep it out of the reach of children.
INACTIVE INGREDIENT SECTION
WATER,1,2-Hexanediol, Sodium Benzoate, PEG-8 CAPRYLIC/CAPRIC GLYCERIDES, PEG-6 CAPRYLIC/CAPRIC GLYCERIDES, POLOXAMER 184, Caprylyl Glycol, POLYSORBATE 20
