Hydroxym
Hydroxym Gel
febffc84-e65e-e811-e053-6294a90a0ad1
HUMAN PRESCRIPTION DRUG LABEL
Jul 12, 2023
PureTek Corporation
DUNS: 785961046
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
HYDROCORTISONE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
Drug Labeling Information
DESCRIPTION SECTION
DESCRIPTION
Each gram of Hydroxym Gel contains 20 mg of hydrocortisone. Inactive ingredients include: Aloe Barbadensis (Aloe Vera) Leaf Juice, Aqua (Purified Water), Hydroxyethyl Cellulose, Methylparaben, PEG-4, Propylene Glycol,Propylparaben.
Chemically, hydrocortisone is [Pregn-4-ene-3,20-dione, 11,17, 21-trihydroxy-, (11β)-] with the molecular formula (C 21H 30O 5) and is represented by the following structural formula:
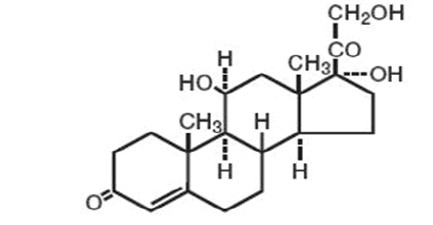
Its molecular weight is 362.47 and its CAS Registery Number is 50-23-7. The topical corticosteroids, including hydrocortisone, constitute a class of primarily synthetic steroids used as anti-inflammatory and antipruritic agents.
HOW SUPPLIED SECTION
HOW SUPPLIED:
Hydroxym™ Gel is available as follows:
1 oz. (28 g) tube (NDC 59088-209-03)
Do not use if tube seal is broken.
** KEEP THIS AND ALL MEDICATION OUT OF THE REACH OF CHILDREN.**
Store at 20-25°C (68-77°F) [see USP Controlled Room Temperature].
