Periogard Alcohol Free
PerioGard (Chlorhexidine Gluconate Oral Rinse USP, 0.12%)
f927717e-e743-43c4-a2b3-d5b83f2403b0
HUMAN PRESCRIPTION DRUG LABEL
Mar 4, 2022
ATLANTIC BIOLOGICALS CORP.
DUNS: 047437707
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Chlorhexidine Gluconate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
PerioGard®
(Chlorhexidine Gluconate
Oral Rinse USP, 0.12%)
ALCOHOL FREE
Rx Only
KEEP OUT OF REACH OF CHILDREN
For questions or comments contact
your dentist or pharmacist.
ADVERSE REACTIONS SECTION
ADVERSE REACTIONS
The most common side effects associated with chlorhexidine gluconate oral rinse USP, 0.12% are: (1) an increase in staining of teeth and other oral surfaces, (2) an increase in calculus formation, and (3) an alteration in taste perception; see WARNINGSand PRECAUTIONS. Oral irritation and local allergy-type symptoms have been spontaneously reported as side effects associated with use of chlorhexidine gluconate rinse. The following oral mucosal side effects were reported during placebo-controlled adult clinical trials: aphthous ulcer, grossly obvious gingivitis, trauma, ulceration, erythema, desquamation, coated tongue, keratinization, geographic tongue, mucocele, and short frenum. Each occurred at a frequency of less than 1.0%.
Among postmarketing reports, the most frequently reported oral mucosal symptoms associated with chlorhexidine gluconate oral rinse USP, 0.12% are stomatitis, gingivitis, glossitis, ulcer, dry mouth, hypesthesia, glossal edema, and paresthesia.
Minor irritation and superficial desquamation of the oral mucosa have been noted in patients using chlorhexidine gluconate oral rinse.
There have been cases of parotid gland swelling and inflammation of the salivary glands (sialadenitis) reported in patients using chlorhexidine gluconate oral rinse.
DESCRIPTION SECTION
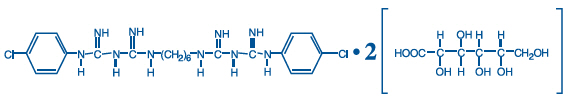
DESCRIPTION
PerioGard® (Chlorhexidine Gluconate Oral rinse USP, 0.12%) is an oral rinse containing 0.12% chlorhexidine gluconate (1,1'-hexamethylene bis [5-(p-chlorophenyl) biguanide] di-D-gluconate) in a base containing water, propylene glycol, glycerin, sorbitol, polyoxyl 40 hydrogenated castor oil, flavor, cetylpyridinium chloride, and FD&C blue no. 1. PerioGard® product is a near neutral solution (pH range 5-7). Chlorhexidine gluconate is a salt of chlorhexidine and gluconic acid. Its chemical structure is: