azithromycin dihydrate
These highlights do not include all the information needed to use AZITHROMYCIN TABLETS safely and effectively. See full prescribing information for AZITHROMYCIN TABLETS.AZITHROMYCIN tablets, 250 mg and 500 mg, for oral useInitial U.S. Approval: 1991
01649d79-4677-4577-a3e4-e054f3f9fc3b
HUMAN PRESCRIPTION DRUG LABEL
Sep 16, 2025
Lupin Pharmaceuticals, Inc.
DUNS: 089153071
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
azithromycin dihydrate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
azithromycin dihydrate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Azithromycin Tablets USP, 250 mg
Container Label-30 Tablets
NDC 68180-861-06
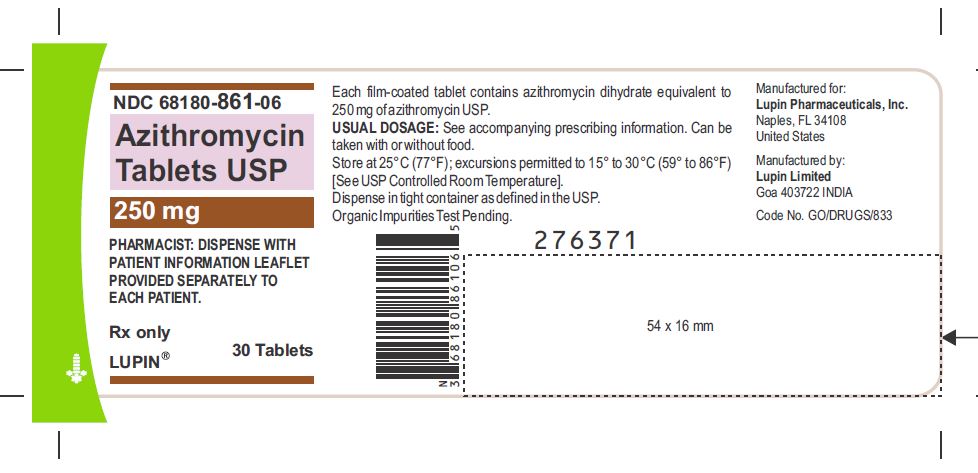
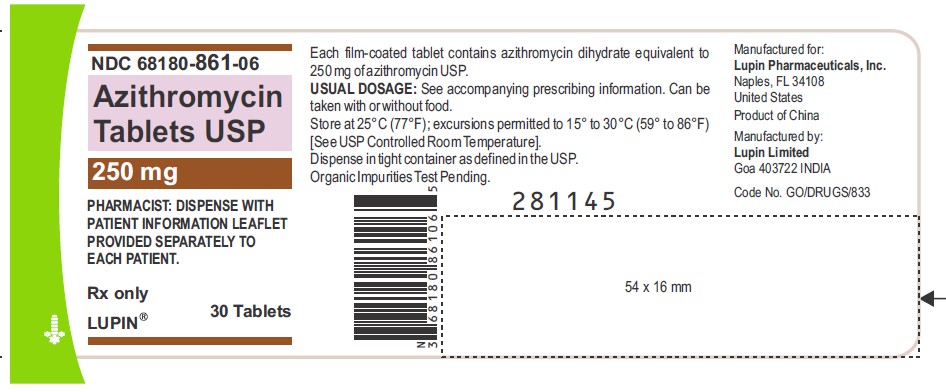
Azithromycin Tablets USP, 250 mg
3 Card x 6 Tablets- Outer Carton Label
NDC 68180-861-13
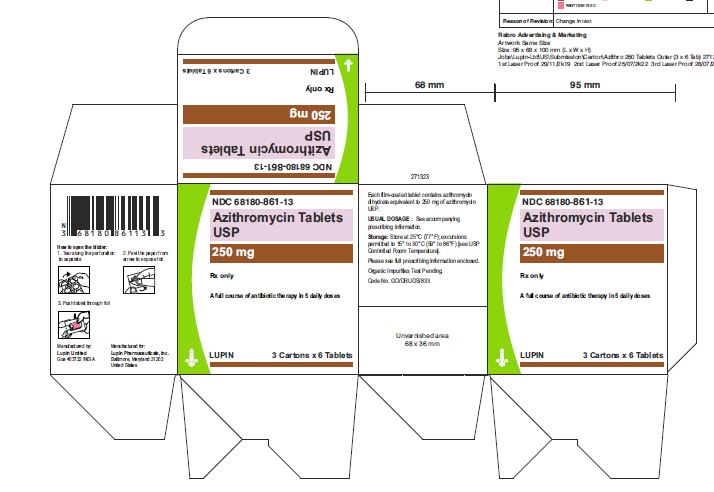
Azithromycin Tablets USP, 250 mg
1 Card x 6 Tablets- Mono Carton Label
NDC 68180-861-11
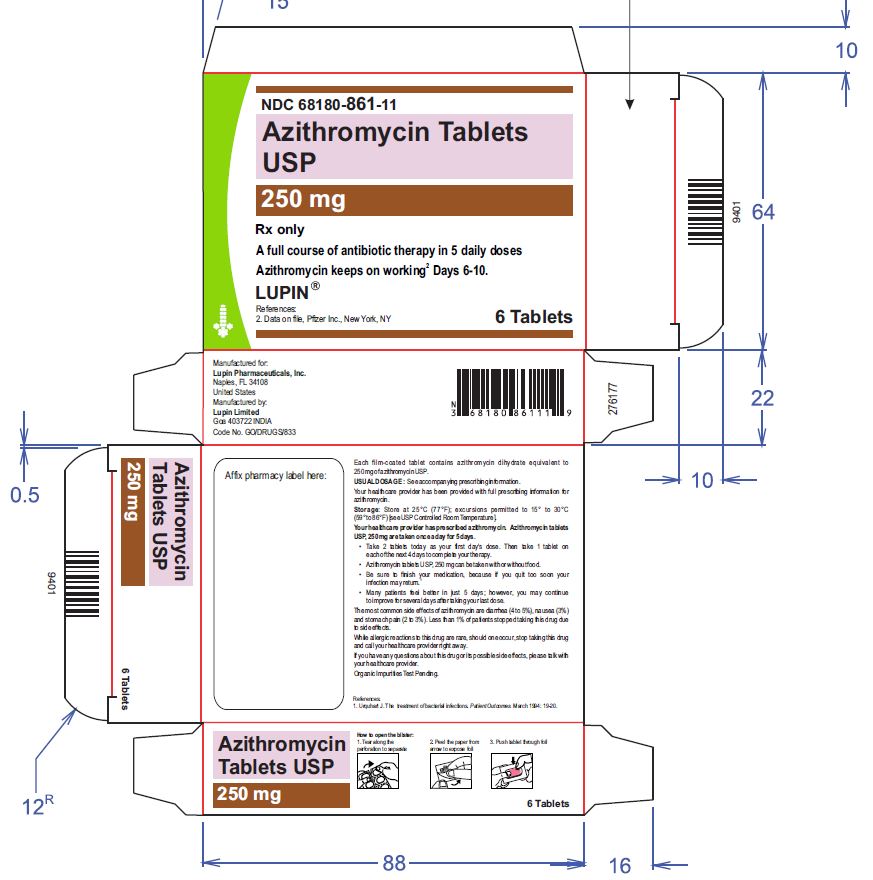
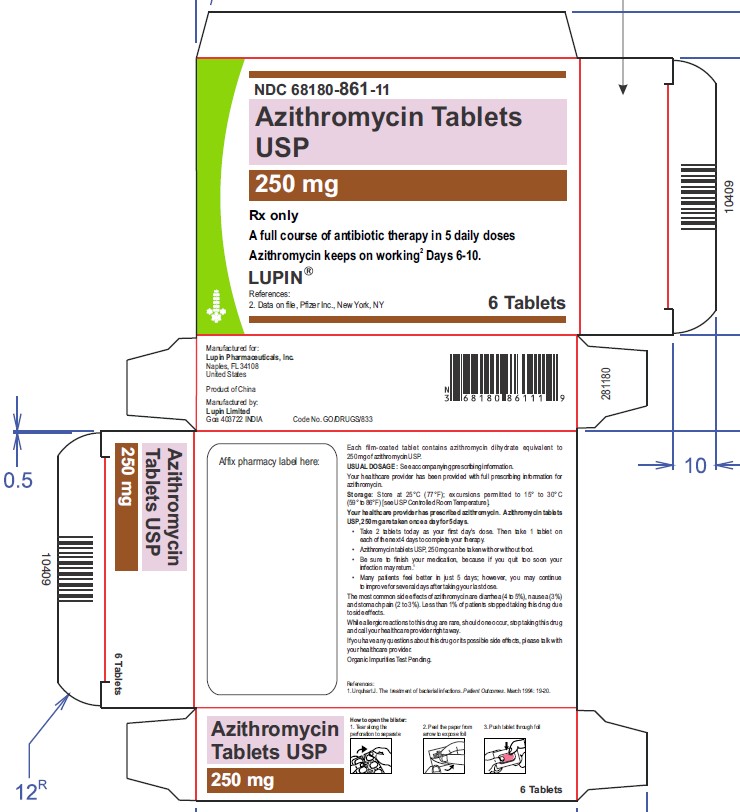
Azithromycin Tablets USP, 250 mg
6 Tablets- Blister Card
NDC 68180-861-11

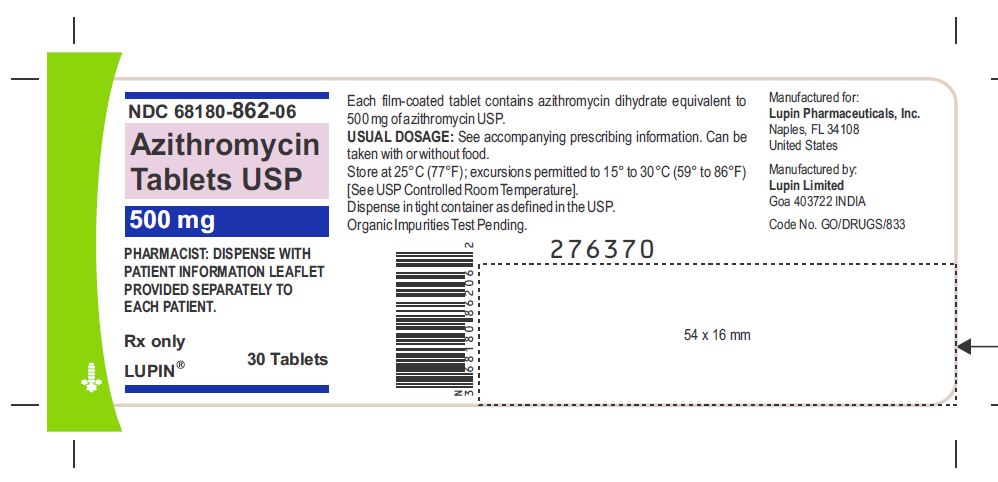
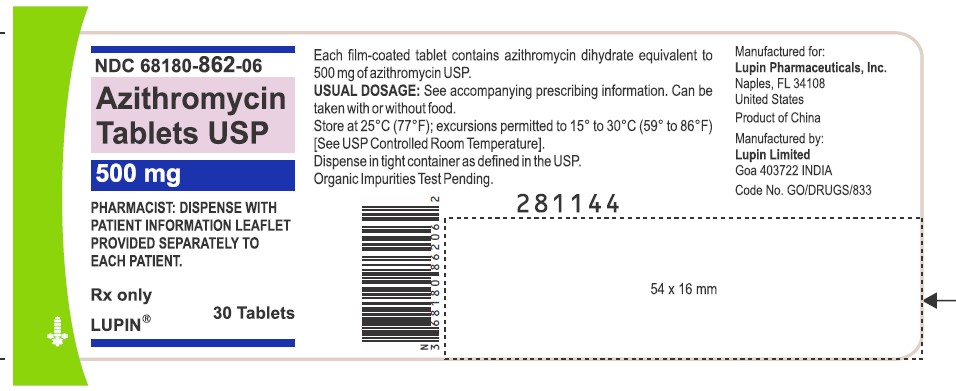
Azithromycin Tablets USP, 500 mg
Container Label-30 Tablets
NDC 68180-862-06
Azithromycin Tablets USP, 500 mg
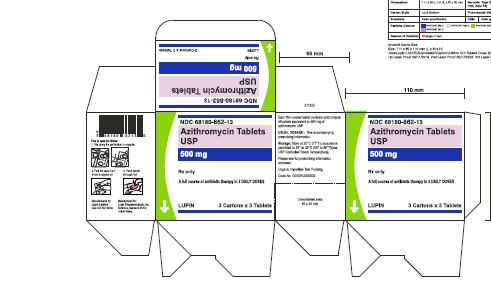
3 Card x 3 Tablets- Outer Carton Label
NDC 68180-862-13
Azithromycin Tablets USP, 500 mg
1 Card x 3 Tablets- Mono Carton Label
NDC 68180-862-11
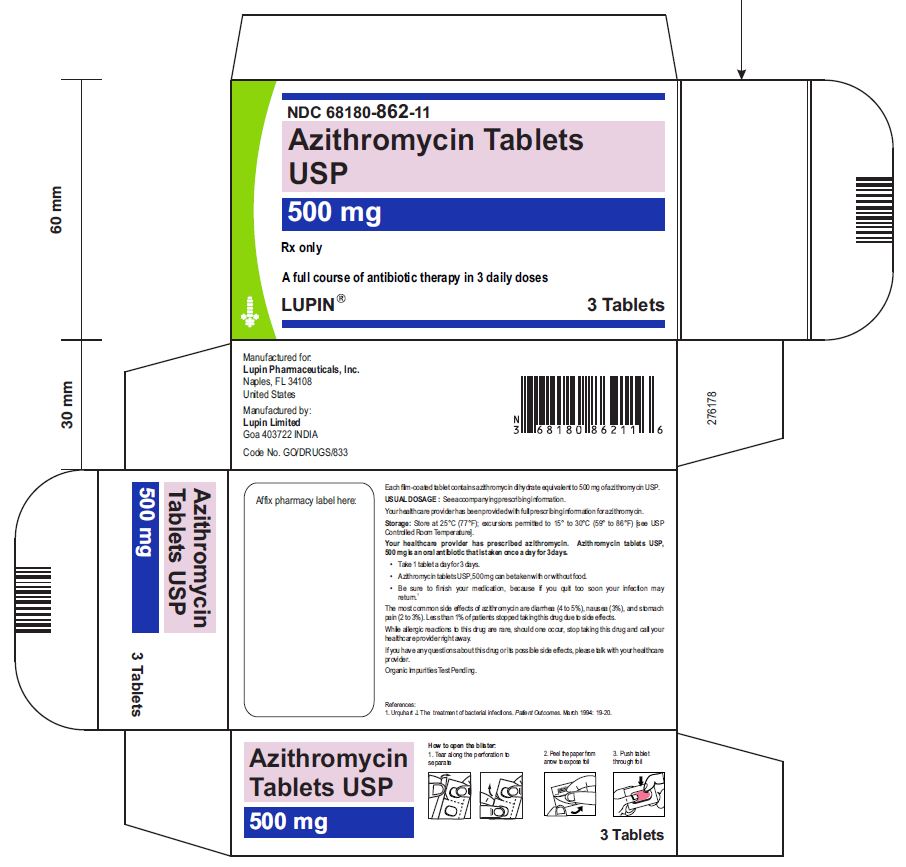
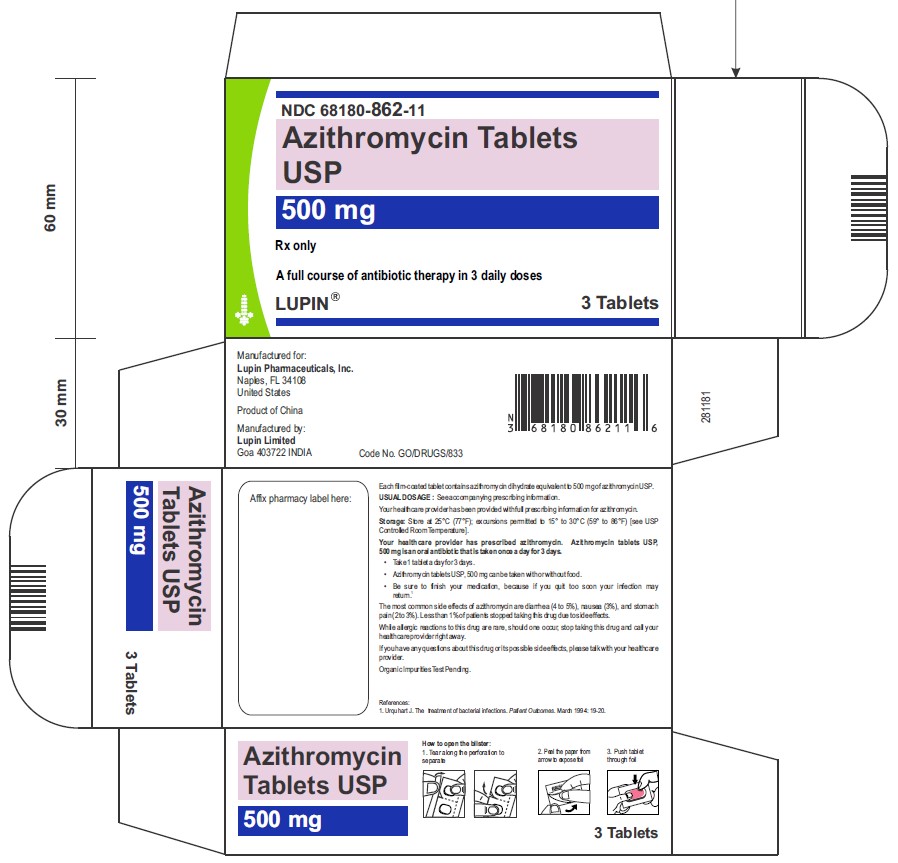
Azithromycin Tablets USP, 500 mg
3 Tablets- Blister Card
NDC 68180-862-11
SPL PATIENT PACKAGE INSERT SECTION
Patient Information
Azithromycin (ay-ZITH-roe-MYE-sin) Tablets USP
Read this Patient Information leaflet before you start taking azithromycin tablets and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
What are azithromycin tablets?
Azithromycin tablets are a macrolide antibiotic prescription medicine used in adults 18 years or older to treat certain infections caused by certain germs called bacteria. These bacterial infections include:
- acute worsening of chronic bronchitis
- acute sinus infection
- community-acquired pneumonia
- infected throat or tonsils
- skin infections
- infections of the urethra or cervix
- genital ulcers in men
Azithromycin tablets are also used in children to treat:
- ear infections
- community-acquired pneumonia
- infected throat or tonsils
Azithromycin should not be taken by people who cannot tolerate oral medications because they are very ill or have certain other risk factors including:
- have cystic fibrosis
- have hospital acquired infections
- have known or suspected bacteria in the blood
- need to be in the hospital
- are elderly
- have any medical problems that can lower the ability of the immune system to fight infections
Azithromycin tablets are not for viral infections such as the common cold.
It is not known if azithromycin tablets are safe and effective for genital ulcers in women.
It is not known if azithromycin tablets are safe and effective for children with ear infections, sinus infections, and community-acquired pneumonia under 6 months of age.
It is not known if azithromycin tablets are safe and effective for infected throat or tonsils in children under 2 years of age.
Who should not take azithromycin tablets?
Do not take azithromycin tablets if you:
- have had a severe allergic reaction to certain antibiotics known as macrolides or ketolides including azithromycin and erythromycin.
- have a history of cholestatic jaundice or hepatic dysfunction that happened with the use of azithromycin.
What should I tell my healthcare provider before taking azithromycin tablets?
**Before you take azithromycin tablets, tell your healthcare provider if you: **
- have pneumonia
- have cystic fibrosis
- have known or suspected bacteremia (bacterial infection in the blood)
- have liver or kidney problems
- have an irregular heartbeat, especially a problem called "QT prolongation"
- have a problem that causes muscle weakness (myasthenia gravis)
- have any other medical problems
- are pregnant or plan to become pregnant. It is not known if azithromycin tablets will harm your unborn baby.
- are breastfeeding or plan to breastfeed. Azithromycin has been reported to pass into breast milk. Talk to your healthcare provider about the best way to feed your baby while you take azithromycin tablets.
Contact your healthcare provider immediately if you are giving azithromycin tablets to a young child (less than 6 weeks of age) and he or she vomits or becomes irritable when fed.
**Tell your healthcare provider about all the medicines you take,**including prescription and non-prescription medicines, vitamins, and herbal supplements.
Azithromycin tablets and other medicines may affect each other causing side effects. Azithromycin tablets may affect the way other medicines work, and other medicines may affect how azithromycin tablets works.
Especially tell your healthcare provider if you take:
- nelfinavir
- a blood thinner (warfarin)
- digoxin
- colchicine
- phenytoin
- an antacid that contains aluminum or magnesium
Know the medicines you take. Keep a list of your medicines and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I take azithromycin tablets?
- Take azithromycin tablets exactly as your healthcare provider tells you to take it.
- Azithromycin tablets can be taken with or without food.
- Do not skip any doses of azithromycin tablets or stop taking it, even if you begin to feel better, until you finish your prescribed treatment unless you have a serious allergic reaction or your healthcare provider tells you to stop taking azithromycin tablets."See What are the possible side effects of azithromycin tablets?" If you skip doses, or do not complete the total course of azithromycin tablets your treatment may not work as well and your infection may be harder to treat. Taking all of your azithromycin tablets doses will help lower the chance that the bacteria will become resistant to azithromycin tablets.
- If the bacteria becomes resistant to azithromycin, azithromycin tablets and other antibiotic medicines may not work for you in the future.
- If you take too much azithromycin tablets, call your healthcare provider or get medical help right away.
What are the possible side effects of azithromycin****tablets?
Azithromycin tablets can cause serious side effects, including:
**• Serious allergic reactions.**Allergic reactions can happen in people taking azithromycin tablets the active ingredient in azithromycin tablets, even after only 1 dose. Stop taking azithromycin tablets and get emergency medical help right away if you have any of the following symptoms of a severe allergic reaction:
° trouble breathing or swallowing
° swelling of the lips, tongue, face
° throat tightness, hoarseness
° rapid heartbeat
° faintness
° skin rash (hives)
° new onset of fever and swollen lymph nodes
Stop taking azithromycin tablets at the first sign of a skin rash and call your healthcare provider.
Skin rash may be a sign of a more serious reaction to azithromycin tablets.
**•****Liver damage (hepatotoxicity).**Hepatotoxicity can happen in people who take azithromycin tablets. Call your healthcare provider right away if you have unexplained symptoms such as:
° nausea or vomiting
° stomach pain
° fever
° weakness
° abdominal pain or tenderness
° itching
° unusual tiredness
° loss of appetite
° change in the color of your bowel movements
° dark colored urine
° yellowing of your skin or of the whites of your eyes
Stop taking azithromycin tablets and tell your healthcare provider right away if you have yellowing of your skin or white part of your eyes, or if you have dark urine. These can be signs of a serious reaction to azithromycin tablets (a liver problem).
•Serious heart rhythm changes that can be life-threatening, including heart stopping (cardiac arrest), QT prolongation, torsades de pointes, feeling that your heart is pounding or racing (palpitations), chest discomfort, or irregular heartbeat.
Tell your healthcare provider right away if you or your child feel a fast or irregular heartbeat, get dizzy or faint. Azithromycin tablets may cause a rare heart problem known as prolongation of the QT interval. This condition can cause an abnormal heartbeat and can be very dangerous. The chances of this happening are higher in people:
° who are elderly
° with a family history of prolonged QT interval
° with low blood potassium
° who take certain medicines to control heart rhythm (antiarrhythmics)
•** Worsening of myasthenia gravis (a problem that causes muscle weakness).**
Certain antibiotics like azithromycin tablets may cause worsening of myasthenia gravis symptoms, including muscle weakness and breathing problems. Call your healthcare provider right away if you have any worsening muscle weakness or breathing problems.
•** Diarrhea.**Tell your healthcare provider right away if you have watery diarrhea, diarrhea that does not go away, or bloody stools. You may experience cramping and a fever. This could happen after you have finished your azithromycin tablets.
The most common side effects of azithromycin tablets include:
•nausea
•stomach pain
•vomiting
These are not all the possible side effects of azithromycin tablets. Tell your healthcare provider about any side effect that bothers you or that does not go away.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store azithromycin tablets?
- Store azithromycin tablets at 15° to 30°C (59° to 86°F).
- Safely throw away any medicine that is out of date or no longer needed.
Keep azithromycin tablets and all medicines out of the reach of children.
General information about the safe and effective use of azithromycin tablets.
Medicines are sometimes prescribed for purposes other than those listed in the Patient Information leaflet. Do not use azithromycin tablets for a condition for which it was not prescribed. Do not give azithromycin tablets to other people, even if they have the same symptoms you have. It may harm them.
This Patient Information leaflet summarizes the most important information about azithromycin tablets. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about azithromycin tablets that is written for health professionals.
For more information, go to www.lupinpharmaceuticals.com or call 1-800-399-2561
What are the ingredients in azithromycin tablets?
Azithromycin Tablets:
Active ingredient: azithromycin dihydrate
Inactive ingredients: croscarmellose sodium, dibasic calcium phosphate, hydroxypropyl methyl cellulose, lactose monohydrate, magnesium stearate, sodium lauryl sulfate, titanium dioxide, triacetin and D & C Red #30.
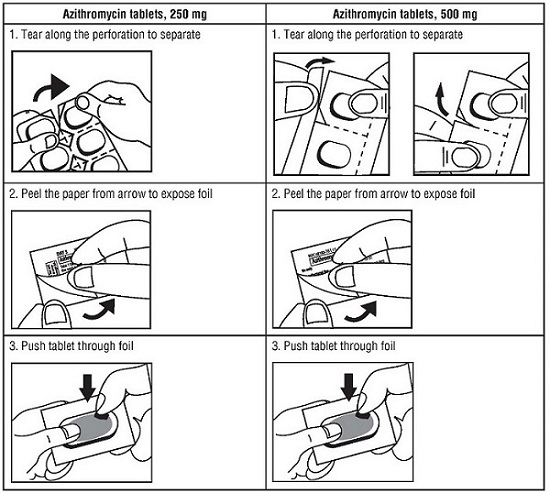
How to open the blister:
This Patient Information has been approved by the U.S. Food and Drug Administration.
LUPIN and the
 are registered trademarks of Lupin Pharmaceuticals, Inc.
are registered trademarks of Lupin Pharmaceuticals, Inc.
Manufactured for:
Lupin Pharmaceuticals, Inc.
Naples, FL 34108
United States
Manufactured by:
Lupin Limited
Goa - 403722
India
Revised: October 2024 ID#: 276374
DESCRIPTION SECTION
11 DESCRIPTION
Azithromycin tablets USP contain the active ingredient azithromycin, a macrolide antibacterial drug, for oral administration. Azithromycin has the chemical name (2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)-13-[(2,6-dideoxy-3-C-methyl-3-O-methyl-α- L-ribo-hexopyranosyl) oxy]-2-ethyl-3,4,10-trihydroxy-3,5,6,8,10,12,14-heptamethyl-11-[[3,4,6-trideoxy-3-(dimethylamino)-β-D- xylo-hexopyranosyl]oxy]-1-oxa-6-azacyclopentadecan-15-one. Azithromycin is derived from erythromycin; however, it differs chemically from erythromycin in that a methyl-substituted nitrogen atom is incorporated into the lactone ring. Its molecular formula is C38H72N2O12, and its molecular weight is 749.00. Azithromycin has the following structural formula:
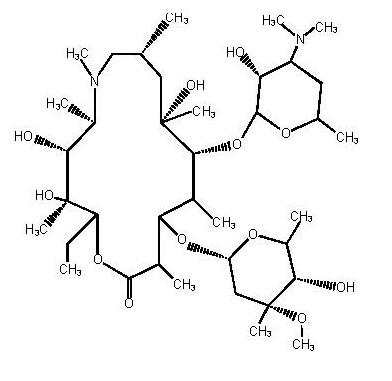
Azithromycin, as the dihydrate, is a white to almost white crystalline powder with a molecular formula of C38H72N2O12•2H2O and a molecular weight of 785.02.
Azithromycin is supplied as tablets containing azithromycin dihydrate equivalent to either 250 mg or 500 mg azithromycin and the following inactive ingredients: croscarmellose sodium, dibasic calcium phosphate, hydroxypropyl methyl cellulose, lactose monohydrate, magnesium stearate, sodium lauryl sulfate, titanium dioxide, triacetin and D&C Red #30.
Organic Impurities Test Pending.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Azithromycin tablets USP are supplied in the following strengths and package configurations:
Azithromycin tablets USP, 250 mg are supplied as pink, oval shaped film-coated tablets, engraved with "LU" on one side and "L04" on the other side containing azithromycin dihydrate equivalent to 250 mg of azithromycin USP.
These are packaged in bottles and blister cards as follows:
Bottles of 30 Tablets NDC 68180-861-06
Carton of 1 Blister Card (6 Tablets per Blister Card) [1 x 6 Tablets] NDC 68180-861-11
Azithromycin tablets USP, 500 mg are supplied as pink, oval shaped film-coated tablets, engraved with "LU" on one side and "L05" on the other side containing azithromycin dihydrate USP equivalent to 500 mg of azithromycin USP.
These are packaged in bottles and blister cards as follows:
Bottles of 30 Tablets NDC 68180-862-06
Carton of 1 Blister Card (3 Tablets per Blister Card) [1 x 3 Tablets] NDC 68180-862-11
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature].
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
General Patient Counseling
Azithromycin tablets can be taken with or without food.
Patients should also be cautioned not to take aluminum-and magnesium- containing antacids and azithromycin simultaneously.
The patient should be directed to discontinue azithromycin immediately and contact a physician if any signs of an allergic reaction occur.
Direct parents or caregivers to contact their physician if vomiting and irritability with feeding occurs in the infant.
Patients should be counseled that antibacterial drugs including azithromycin should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When azithromycin is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of the therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by azithromycin or other antibacterial drugs in the future.
Diarrhea is a common problem caused by antibacterials which usually ends when the antibacterial is discontinued. Sometimes after starting treatment with antibacterials patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibacterial drug. If this occurs, patients should contact their physician as soon as possible.
LUPIN and the
 are registered trademarks of Lupin Pharmaceuticals, Inc.
are registered trademarks of Lupin Pharmaceuticals, Inc.
Manufactured for:
Lupin Pharmaceuticals, Inc.
Naples, FL 34108
United States
Manufactured by:
Lupin Limited
Goa - 403722
India
Revised: October 2024
