Deferasirox
These highlights do not include all the information needed to use DEFERASIROX TABLETS FOR ORAL SUSPENSION safely and effectively. See full prescribing information for DEFERASIROX TABLETS FOR ORAL SUSPENSION. DEFERASIROX tablets, for oral suspension Initial U.S. Approval: 2005
ad73cb1f-41d6-4cde-9abc-fc2cb7e04c95
HUMAN PRESCRIPTION DRUG LABEL
Aug 31, 2021
Actavis Pharma, Inc.
DUNS: 119723554
Products 3
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Deferasirox
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
Deferasirox
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
Deferasirox
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
Drug Labeling Information
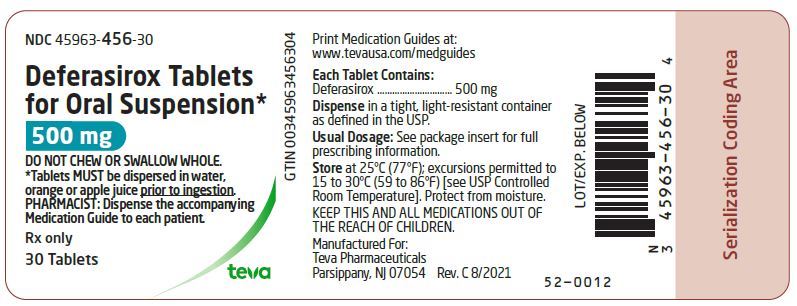
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NDC 45963-456-30
Deferasirox Tablets for Oral Suspension*
500 mg
DO NOT CHEW OR SWALLOW WHOLE.
- Tablets MUST be dispersed in water, orange or apple juice prior to ingestion.
PHARMACIST: Dispense the accompanying Medication Guide to each patient.
Rx only
30 Tablets
BOXED WARNING SECTION
**WARNING: RENAL FAILURE, HEPATIC FAILURE, and GASTROINTESTINAL
HEMORRHAGE**
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
1.1 Treatment of Chronic Iron Overload Due to Blood Transfusions
(Transfusional Iron Overload)
Deferasirox tablets for oral suspension are indicated for the treatment of chronic iron overload due to blood transfusions (transfusional hemosiderosis) in patients 2 years of age and older.
1.2 Treatment of Chronic Iron Overload in Non-Transfusion-Dependent
Thalassemia Syndromes
Deferasirox tablets for oral suspension are indicated for the treatment of chronic iron overload in patients 10 years of age and older with non- transfusion-dependent thalassemia (NTDT) syndromes and with a liver iron concentration (LIC) of at least 5 milligrams of iron per gram of liver dry weight (mg Fe/g dw) and a serum ferritin greater than 300 mcg/L.
1.3 Limitations of Use
The safety and efficacy of deferasirox when administered with other iron chelation therapy have not been established.
Deferasirox tablets for oral suspension are an iron chelator indicated for the treatment of chronic iron overload due to blood transfusions in patients 2 years of age and older. (1.1)
Deferasirox tablets for oral suspension are indicated for the treatment of chronic iron overload in patients 10 years of age and older with non- transfusion-dependent thalassemia (NTDT) syndromes, and with a liver iron (Fe) concentration (LIC) of at least 5 mg Fe per gram of dry weight and a serum ferritin greater than 300 mcg/L. (1.2)
Limitations of Use:
The safety and efficacy of deferasirox when administered with other iron chelation therapy have not been established. (1.3)
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
Deferasirox is contraindicated in patients with:
- Estimated GFR less than 40 mL/min/1.73 m2 [see Dosage and Administration (2.5), Warnings and Precautions (5.1)];
- Poor performance status; [see Warnings and Precautions (5.1, 5.3)]
- High-risk myelodysplastic syndromes; (this patient population was not studied and is not expected to benefit from chelation therapy)
- Advanced malignancies; [see Warnings and Precautions (5.1, 5.3)]
- Platelet counts less than 50 x 109/L; [see Warnings and Precautions (5.3, 5.4)]
- Known hypersensitivity to deferasirox or any component of deferasirox [see Warnings and Precautions (5.7), Adverse Reactions (6.2)].
- Estimated GFR less than 40 mL/min/1.73 m2. (4)
- Patients with poor performance status. (4)
- Patients with high-risk myelodysplastic syndrome (MDS). (4)
- Patients with advanced malignancies. (4)
- Patients with platelet counts less than 50 x 109/L. (4)
- Known hypersensitivity to deferasirox or any component of deferasirox. (4)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are also discussed in other sections of the labeling:
- Acute Kidney Injury, Including Acute Renal Failure Requiring Dialysis, and Renal Tubular Toxicity Including Fanconi Syndrome [see Warnings and Precautions (5.1, 5.6)]
- Hepatic Toxicity and Failure [see Warnings and Precautions (5.2, 5.6)]
- GI Hemorrhage [see Warnings and Precautions (5.3)]
- Bone Marrow Suppression [see Warnings and Precautions (5.4)]
- Hypersensitivity [see Warnings and Precautions (5.7)]
- Severe Skin Reactions [see Warnings and Precautions (5.8)]
- Skin Rash [see Warnings and Precautions (5.9)]
- Auditory and Ocular Abnormalities [see Warnings and Precautions (5.10)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Transfusional Iron Overload
A total of 700 adult and pediatric patients were treated with deferasirox for 48 weeks in premarketing studies. These included 469 patients with beta- thalassemia, 99 with rare anemias, and 132 with sickle cell disease. Of these patients, 45% were male, 70% were Caucasian, and 292 patients were less than 16 years of age. In the sickle cell disease population, 89% of patients were black. Median treatment duration among the sickle cell patients was 51 weeks. Of the 700 patients treated, 469 (403 beta-thalassemia and 66 rare anemias) were entered into extensions of the original clinical protocols. In ongoing extension studies, median durations of treatment were 88 to 205 weeks.
Six hundred twenty-seven (627) patients with myelodysplastic syndrome (MDS) were enrolled across 5 uncontrolled trials. These studies varied in duration from 1 to 5 years. The discontinuation rate across studies in the first year was 46% (adverse events 20%, withdrawal of consent 10%, death 8%, other 4%, lab abnormalities 3%, and lack of efficacy 1%). Among 47 patients enrolled in the study of 5-year duration, 10 remained on deferasirox at the completion of the study.
Table 1 displays adverse reactions occurring in greater than 5% of deferasirox-treated beta-thalassemia patients (Study 1), sickle cell disease patients (Study 3), and patients with MDS (MDS pool). Abdominal pain, nausea, vomiting, diarrhea, skin rashes, and increases in serum creatinine were the most frequent adverse reactions reported with a suspected relationship to deferasirox. Gastrointestinal symptoms, increases in serum creatinine, and skin rash were dose related.
Table 1. Adverse Reactionsa Occurring in Greater Than 5% of Deferasirox-treated Patients in Study 1, Study 3, and MDS Pool|
Study 1 |
Study 3 | ||||
|
(Beta-thalassemia) |
(Sickle Cell Disease) |
MDS Pool | |||
|
Deferasirox |
Deferoxamine |
Deferasirox |
Deferoxamine |
Deferasirox | |
|
N = 296 |
N = 290 |
N = 132 |
N = 63 |
N = 627 | |
|
Adverse Reactions |
n (%) |
n (%) |
n (%) |
n (%) |
n (%) |
|
Abbreviation: MDS, myelodysplastic syndrome. | |||||
|
aAdverse reaction frequencies are based on adverse events reported regardless of relationship to study drug. | |||||
|
bIncludes ‘abdominal pain’, ‘abdominal pain lower’, and ‘abdominal pain upper’. | |||||
|
cIncludes ‘blood creatinine increased’ and ‘blood creatinine abnormal’. See also Table 2. | |||||
|
Abdominal Painb |
63 (21) |
41 (14) |
37 (28) |
9 (14) |
145 (23) |
|
Diarrhea |
35 (12) |
21 (7) |
26 (20) |
3 (5) |
297 (47) |
|
Creatinine Increasedc |
33 (11) |
0 (0) |
9 (7) |
0 |
89 (14) |
|
Nausea |
31 (11) |
14 (5) |
30 (23) |
7 (11) |
161 (26) |
|
Vomiting |
30 (10) |
28 (10) |
28 (21) |
10 (16) |
83 (13) |
|
Rash |
25 (8) |
9 (3) |
14 (11) |
3 (5) |
83 (13) |
In Study 1, a total of 113 (38%) patients treated with deferasirox had increases in serum creatinine greater than 33% above baseline on 2 separate occasions (Table 2) and 25 (8%) patients required dose reductions. Increases in serum creatinine appeared to be dose related [see Warnings and Precautions (5.1)]. In this study, 17 (6%) patients treated with deferasirox developed elevations in serum glutamic-pyruvic transaminase (SGPT)/ALT levels greater than 5 times the upper limit of normal (ULN) at 2 consecutive visits. Of these, 2 patients had liver biopsy proven drug-induced hepatitis and both discontinued deferasirox therapy [see Warnings and Precautions (5.2)]. An additional 2 patients, who did not have elevations in SGPT/ALT greater than 5 times the ULN, discontinued deferasirox because of increased SGPT/ALT. Increases in transaminases did not appear to be dose related. Adverse reactions that led to discontinuations included abnormal liver function tests (2 patients) and drug-induced hepatitis (2 patients), skin rash, glycosuria/proteinuria, Henoch Schönlein purpura, hyperactivity/insomnia, drug fever, and cataract (1 patient each).
In Study 3, a total of 48 (36%) patients treated with deferasirox had increases in serum creatinine greater than 33% above baseline on 2 separate occasions (Table 2) [see Warnings and Precautions (5.1)]. Of the patients who experienced creatinine increases in Study 3, 8 deferasirox-treated patients required dose reductions. In this study, 5 patients in the deferasirox group developed elevations in SGPT/ALT levels greater than 5 times the ULN at 2 consecutive visits and 1 patient subsequently had deferasirox permanently discontinued. Four additional patients discontinued deferasirox due to adverse reactions with a suspected relationship to study drug, including diarrhea, pancreatitis associated with gallstones, atypical tuberculosis, and skin rash.
In the MDS pool, in the first year, a total of 229 (37%) patients treated with deferasirox had increases in serum creatinine greater than 33% above baseline on 2 consecutive occasions (Table 2) and 8 (3.5%) patients permanently discontinued [see Warnings and Precautions (5.1)]. A total of 5 (0.8%) patients developed SGPT/ALT levels greater than 5 times the ULN at 2 consecutive visits. The most frequent adverse reactions that led to discontinuation included increases in serum creatinine, diarrhea, nausea, rash, and vomiting. Death was reported in the first year in 52 (8%) of patients [see Clinical Studies (14)].
Table 2. Number (%) of Patients with Increases in Serum Creatinine or SGPT/ALT in Study 1, Study 3, and MDS Pool|
Study 1 (Beta-thalassemia) |
Study 3 (Sickle Cell Disease) |
MDS Pool | |||
|
Deferasirox |
Deferoxamine |
Deferasirox |
Deferoxamine |
Deferasirox | |
|
N = 296 |
N = 290 |
N = 132 |
N = 63 |
N = 627 | |
|
Laboratory Parameter |
n (%) |
n (%) |
n (%) |
n (%) |
n (%) |
|
Serum Creatinine | |||||
|
Creatinine increase > 33% at 2 consecutive post-baseline visits |
113 (38) |
41 (14) |
48 (36) |
14 (22) |
229 (37) |
|
Creatinine increase > 33% and > ULN at 2 consecutive post-baseline visits |
7 (2) |
1 (0) |
3 (2) |
2 (3) |
126 (20) |
|
SGPT/ALT | |||||
|
SGPT/ALT > 5 x ULN at 2 post-baseline visits |
25 (8) |
7 (2) |
2 (2) |
0 |
9 (1) |
|
SGPT/ALT > 5 x ULN at 2 consecutive post-baseline visits |
17 (6) |
5 (2) |
5 (4) |
0 |
5 (1) |
|
Abbreviations: ALT, alanine transaminase; MDS, myelodysplastic syndrome; SGPT, serum glutamic-pyruvic transaminase; ULN, upper limit of normal. |
Non-Transfusion-Dependent Thalassemia Syndromes
In Study 5, 110 patients with NTDT received 1 year of treatment with deferasirox 5 or 10 mg/kg/day and 56 patients received placebo in a double- blind, randomized trial. In Study 6, 130 of the patients who completed Study 5 were treated with open-label deferasirox at 5, 10, or 20 mg/kg/day (depending on the baseline LIC) for 1 year [see Clinical Studies (14)]. Tables 3 and 4 display the frequency of adverse reactions in patients with NTDT. Adverse reactions with a suspected relationship to study drug were included in Table 3 if they occurred at ≥ 5% of patients in Study 5.
Table 3. Adverse Reactions Occurring in Greater Than 5% in Patients with NTDT|
Study 5 |
Study 6 | ||
|
Deferasirox |
Placebo |
Deferasirox | |
|
N = 110 |
N = 56 |
N = 130 | |
|
n (%) |
n (%) |
n (%) | |
|
Any adverse reaction |
31 (28) |
** 9 (16)** |
27 (21) |
|
Nausea |
7 (6) |
4 (7) |
2 (2)a |
|
Rash |
7 (6) |
1 (2) |
2 (2)a |
|
Diarrhea |
5 (5) |
1 (2) |
7 (5) |
|
Abbreviation: NTDT, non-transfusion-dependent thalassemia. | |||
|
a The occurrence of nausea, and rash are included for Study 6. There were no additional adverse reactions with a suspected relationship to study drug occurring in greater than 5% of patients in Study 6. |
In Study 5, 1 patient in the placebo 10 mg/kg/day group experienced an ALT increase to greater than 5 times ULN and greater than 2 times baseline (Table 4). Three deferasirox-treated patients (all in the 10 mg/kg/day group) had 2 consecutive serum creatinine level increases greater than 33% from baseline and greater than ULN. Serum creatinine returned to normal in all 3 patients (in 1 spontaneously and in the other 2 after drug interruption). Two additional cases of ALT increase and 2 additional cases of serum creatinine increase were observed in the 1-year extension of Study 5. The number (%) of patients with NTDT with increase in serum creatinine or SGPT/ALT in Study 5 and Study 6 are presented in Table 4 below.
Table 4. Number (%) of Patients with NTDT with Increases in Serum Creatinine or SGPT/ALT|
Study 5 |
Study 6 | ||
|
Deferasirox |
Placebo |
Deferasirox | |
|
N = 110 |
N = 56 |
N = 130 | |
|
Laboratory Parameter |
n (%) |
n (%) |
n (%) |
|
Serum creatinine (> 33% increase from baseline and > ULN at ≥2 consecutive post-baseline values) |
3 (3) |
0 |
2 (2) |
|
SGPT/ALT (> 5 x ULN and > 2 x baseline) |
1 (1) |
1 (2) |
2 (2) |
|
Abbreviations: ALT, alanine transaminase; NTDT, non-transfusion-dependent thalassemia; SGPT, serum glutamic-pyruvic transaminase; ULN, upper limit of normal. |
Proteinuria
In clinical studies, urine protein was measured monthly. Intermittent proteinuria (urine protein/creatinine ratio greater than 0.6 mg/mg) occurred in 18.6% of deferasirox-treated patients compared to 7.2% of deferoxamine- treated patients in Study 1 [see Warnings and Precautions (5.1)].
Other Adverse Reactions
In the population of more than 5,000 patients with transfusional iron overload who have been treated with deferasirox during clinical trials, adverse reactions occurring in 0.1% to 1% of patients included gastritis, edema, sleep disorder, pigmentation disorder, dizziness, anxiety, maculopathy, cholelithiasis, pyrexia, fatigue, laryngeal pain, cataract, hearing loss, GI hemorrhage, gastric ulcer (including multiple ulcers), duodenal ulcer, renal tubular disorder (Fanconi Syndrome), and acute pancreatitis (with and without underlying biliary conditions). Adverse reactions occurring in 0.01% to 0.1% of patients included optic neuritis, esophagitis, erythema multiforme, and drug reaction with eosinophilia and systemic symptoms (DRESS). Adverse reactions, which most frequently led to dose interruption or dose adjustment during clinical trials were rash, GI disorders, infections, increased serum creatinine, and increased serum transaminases.
Pooled Analysis of Pediatric Clinical Trial Data
A nested case control analysis was conducted within a deferasirox tablets for oral suspension pediatric pooled clinical trial dataset to evaluate the effects of dose and serum ferritin level, separately and combined, on kidney function. Among 1213 children (aged 2 to 15 years) with transfusion-dependent thalassemia, 162 cases of acute kidney injury (eGFR ≤ 90 mL/min/1.73 m2) and 621 matched-controls with normal kidney function (eGFR ≥ 120 mL/min/1.73 m2) were identified. The primary findings were:
- A 26% increased risk of acute kidney injury was observed with each 5 mg/kg
increase in daily deferasirox dosage starting at 20 mg/kg/day
(95% confidence interval (CI): 1.08 to 1.48).
- A 25% increased risk for acute kidney injury was observed with each 250
mcg/L decrease in serum ferritin starting at 1250 mcg/L (95% CI:
1.01 to 1.56).
- Among pediatric patients with a serum ferritin < 1,000 mcg/L, those who
received deferasirox dosage > 30 mg/kg/day, compared to those who
received lower dosages, had a higher risk for acute kidney injury (Odds ratio
(OR) = 4.47, 95% CI: 1.25 to 15.95), consistent with overchelation.
In addition, a cohort based analysis of ARs was conducted in the deferasirox tablets for oral suspension pediatric pooled clinical trial data. Pediatric patients who received deferasirox dose > 25 mg/kg/day when their serum ferritin was < 1,000 mcg/L (n = 158) had a 6-fold greater rate of renal adverse reactions (incidence rate ration (IRR) = 6.00, 95% CI: 1.75 to 21.36) and a 2-fold greater rate of dose interruptions (IRR = 2.06, 95% CI: 1.33 to 3.17) compared to the time-period prior to meeting these simultaneous criteria. Adverse reaction of special interest (cytopenia, renal, hearing, and GI disorders) occurred 1.9-fold more frequently when these simultaneous criteria were met, compared to preceding time-periods (IRR = 1.91, 95% CI: 1.05 to 3.48) [see Warnings and Precautions (5.6)].
Additional pediatric use information is approved for Novartis Pharmaceuticals Corporation’s EXJADE® (deferasirox) tablets for oral suspension. However, due to Novartis Pharmaceuticals Corporation’s marketing exclusivity rights, this drug product is not labeled with that information.
6.2 Postmarketing Experience
The following adverse reactions have been spontaneously reported during postapproval use of deferasirox in the transfusional-iron overload setting. Because these reactions are reported voluntarily from a population of uncertain size, in which patients may have received concomitant medication, it is not always possible to reliably estimate frequency or establish a causal relationship to drug exposure.
Skin and Subcutaneous Tissue Disorders: Stevens-Johnson syndrome (SJS), hypersensitivity vasculitis, urticaria, alopecia, toxic epidermal necrolysis (TEN)
Immune System Disorders: hypersensitivity reactions (including anaphylactic reaction and angioedema)
Renal and Urinary Disorders: acute renal failure, tubulointerstitial nephritis
Hepatobiliary Disorders: hepatic failure
Gastrointestinal Disorders: GI perforation
Blood and Lymphatic System Disorders: worsening anemia
5-Year Pediatric Registry
In a 5-year observational study, 267 pediatric patients 2 to < 6 years of age (at enrollment) with transfusional hemosiderosis received deferasirox. Of the 242 patients who had pre- and post-baseline eGFR measurements, 116 (48%) patients had a decrease in eGFR of ≥ 33% observed at least once. Twenty-one (18%) of these 116 patients with decreased eGFR had a dose interruption, and 15 (13%) of these 116 patients had a dose decrease within 30 days. Adverse reactions leading to permanent discontinuation from the study included liver injury (n = 11), renal tubular disorder (n = 1), proteinuria (n = 1), hematuria (n = 1), upper GI hemorrhage (n = 1), vomiting (n = 2), abdominal pain (n = 1), and hypokalemia (n = 1).
In patients with transfusional iron overload, the most frequently occurring (greater than 5%) adverse reactions are diarrhea, vomiting, nausea, abdominal pain, skin rashes, and increases in serum creatinine. In deferasirox-treated patients with NTDT syndromes, the most frequently occurring (greater than 5%) adverse reactions are diarrhea, rash, and nausea. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Teva at 1-888-838-2872 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Aluminum-Containing Antacid Preparations
The concomitant administration of deferasirox and aluminum-containing antacid preparations has not been formally studied. Although deferasirox has a lower affinity for aluminum than for iron, do not take deferasirox with aluminum- containing antacid preparations due to the mechanism of action of deferasirox.
7.2 Agents Metabolized by CYP3A4
Deferasirox may induce CYP3A4 resulting in a decrease in CYP3A4 substrate concentration when these drugs are coadministered. Closely monitor patients for signs of reduced effectiveness when deferasirox is administered with drugs metabolized by CYP3A4 (e.g., alfentanil, aprepitant, budesonide, buspirone, conivaptan, cyclosporine, darifenacin, darunavir, dasatinib, dihydroergotamine, dronedarone, eletriptan, eplerenone, ergotamine, everolimus, felodipine, fentanyl, hormonal contraceptive agents, indinavir, fluticasone, lopinavir, lovastatin, lurasidone, maraviroc, midazolam, nisoldipine, pimozide, quetiapine, quinidine, saquinavir, sildenafil, simvastatin, sirolimus, tacrolimus, tolvaptan, tipranavir, triazolam, ticagrelor, and vardenafil) [see Clinical Pharmacology (12.3)].
7.3 Agents Metabolized by CYP2C8
Deferasirox inhibits CYP2C8 resulting in an increase in CYP2C8 substrate (e.g., repaglinide and paclitaxel) concentration when these drugs are coadministered. If deferasirox and repaglinide are used concomitantly, consider decreasing the dose of repaglinide and perform careful monitoring of blood glucose levels. Closely monitor patients for signs of exposure related toxicity when deferasirox is coadministered with other CYP2C8 substrates [see Clinical Pharmacology (12.3)].
7.4 Agents Metabolized by CYP1A2
Deferasirox inhibits CYP1A2 resulting in an increase in CYP1A2 substrate (e.g., alosetron, caffeine, duloxetine, melatonin, ramelteon, tacrine, theophylline, tizanidine) concentration when these drugs are coadministered. An increase in theophylline plasma concentrations could lead to clinically significant theophylline-induced CNS or other adverse reactions. Avoid the concomitant use of theophylline or other CYP1A2 substrates with a narrow therapeutic index (e.g., tizanidine) with deferasirox. Monitor theophylline concentrations and consider theophylline dose modification if you must coadminister theophylline with deferasirox. Closely monitor patients for signs of exposure related toxicity when deferasirox is coadministered with other drugs metabolized by CYP1A2 [see Clinical Pharmacology (12.3)].
7.5 Agents Inducing UDP-glucuronosyltransferase (UGT) Metabolism
Deferasirox is a substrate of UGT1A1 and to a lesser extent UGT1A3. The concomitant use of deferasirox with potent UGT inducers (e.g., rifampicin, phenytoin, phenobarbital, ritonavir) may result in a decrease in deferasirox efficacy due to a possible decrease in deferasirox concentration. Avoid the concomitant use of potent UGT inducers with deferasirox. Consider increasing the initial dose of deferasirox if you must coadminister these agents together [see Dosage and Administration (2.5), Clinical Pharmacology (12.3)].
7.6 Bile Acid Sequestrants
Avoid the concomitant use of bile acid sequestrants (e.g., cholestyramine, colesevelam, colestipol) with deferasirox due to a possible decrease in deferasirox concentration. If you must coadminister these agents together, consider increasing the initial dose of deferasirox [see Dosage and Administration (2.5), Clinical Pharmacology (12.3)].
7.7 Busulfan
Increased exposure of busulfan was observed with concomitant use with deferasirox. Monitor plasma concentrations of busulfan when coadministered with deferasirox to allow dose adjustment of busulfan as needed [see Clinical Pharmacology (12.3)].
- Do not take deferasirox with aluminum-containing antacid preparations. (7.1)
- Deferasirox increases the exposure of the CYP2C8 substrate repaglinide. Consider repaglinide dose reduction and monitor blood glucose levels. (7.3)
- Avoid the use of deferasirox with CYP1A2 substrate theophylline. (7.4)
- Deferasirox increases exposure of busulfan. Monitor plasma concentrations of busulfan when coadministered with deferasirox to allow dose adjustment of busulfan as needed. (7.7)
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Transfusional Iron Overload
Deferasirox therapy should only be considered when a patient has evidence of chronic transfusional iron overload. The evidence should include the transfusion of at least 100 mL/kg of packed red blood cells (e.g., at least 20 units of packed red blood cells for a 40 kg person or more in individuals weighing more than 40 kg), and a serum ferritin consistently greater than 1,000 mcg/L.
Prior to starting therapy or increasing dose, evaluate:
-
Serum ferritin level
-
Baseline renal function:
- Obtain serum creatinine in duplicate (due to variations in measurements) to establish accurate baseline
- Calculate the estimated glomerular filtration rate (eGFR). Use a prediction equation appropriate for adult patients (e.g., CKD-EPI, MDRD method) and in pediatric patients (e.g., Schwartz equations).
- Obtain urinalyses and serum electrolytes to evaluate renal tubular function [see Dosage and Administration (2.4), Warnings and Precautions (5.1)].
-
Serum transaminases and bilirubin [see Dosage and Administration (2.4), Warnings and Precautions (5.2)]
-
Baseline auditory and ophthalmic examinations [see Warnings and Precautions (5.10)]
Initiating Therapy:
The recommended initial dose of deferasirox for patients 2 years of age and older with eGFR greater than 60 mL/min/1.73 m2 is 20 mg per kg body weight orally, once daily. Calculate doses (mg per kg per day) to the nearest whole tablet.
During Therapy:
-
Monitor serum ferritin monthly and adjust the dose of deferasirox, if necessary, every 3 to 6 months based on serum ferritin trends.
-
Use the minimum effective dose to achieve a trend of decreasing ferritin.
-
Make dose adjustments in steps of 5 or 10 mg per kg and tailor adjustments to the individual patient’s response and therapeutic goals.
-
In patients not adequately controlled with doses of 30 mg per kg (e.g., serum ferritin levels persistently above 2,500 mcg/L and not showing a decreasing trend over time), doses of up to 40 mg per kg may be considered. Doses above 40 mg per kg are not recommended [see Warnings and Precautions (5.6)].
-
Adjust dose based on serum ferritin levels
-
If the serum ferritin falls below 1,000 mcg/L at 2 consecutive visits, consider dose reduction, especially if the dose is greater than 25 mg/kg/day [see Adverse Reactions (6.1)].
-
If the serum ferritin falls below 500 mcg/L, interrupt deferasirox to minimize the risk of overchelation, and continue monthly monitoring [see Warnings and Precautions (5.6)].
-
Evaluate the need for ongoing chelation therapy for patients whose conditions no longer require regular blood transfusions.
-
Use the minimum effective dose to maintain iron burden in the target range [see Warnings and Precautions (5.6)].
-
-
Monitor blood counts, liver function, renal function and ferritin monthly [see Warnings and Precautions (5.1, 5.2, 5.4)].
-
Interrupt deferasirox for pediatric patients who have acute illnesses, which can cause volume depletion, such as vomiting, diarrhea, or prolonged decreased oral intake, and monitor more frequently. Resume therapy as appropriate, based on assessments of renal function, when oral intake and volume status are normal [see Dosage and Administration (2.4, 2.5), Warnings and Precautions (5.1), Use in Specific Populations (8.4), Clinical Pharmacology (12.3)].
2.2 Iron Overload in Non-Transfusion-Dependent Thalassemia Syndromes
Deferasirox therapy should only be considered when a patient with NTDT syndrome has an LIC of at least 5 mg Fe/g dw and a serum ferritin greater than 300 mcg/L.
Prior to starting therapy, obtain:
-
LIC by liver biopsy or by an FDA-cleared or approved method for identifying patients for treatment with deferasirox therapy
-
Serum ferritin level on at least 2 measurements 1-month apart [see Clinical Studies (14)]
-
Baseline renal function:
-
Obtain serum creatinine in duplicate (due to variations in measurements) to establish accurate baseline
-
Calculate eGFR. Use a prediction equation appropriate for adult patients (e.g., CKD-EPI, MDRD method) and in pediatric patients (e.g., Schwartz equations).
-
Obtain urinalyses and serum electrolytes to evaluate renal tubular function [see Dosage and Administration (2.4), Warnings and Precautions (5.1)].
-
Serum transaminases and bilirubin [see Dosage and Administration (2.4), Warnings and Precautions (5.2)]
-
Baseline auditory and ophthalmic examinations [see Warnings and Precautions (5.10)]
Initiating Therapy:
- The recommended initial dose of deferasirox for patients with eGFR greater than 60 mL/min/1.73 m2 is 10 mg per kg body weight orally once daily. Calculate doses (mg per kg per day) to the nearest whole tablet.
- If the baseline LIC is greater than 15 mg Fe/g dw, consider increasing the dose to 20 mg/kg/day after 4 weeks.
During Therapy:
- Monitor serum ferritin monthly to assess the patient’s response to therapy and to minimize the risk of overchelation [see Warnings and Precautions (5.6)]. Interrupt treatment when serum ferritin is less than 300 mcg/L and obtain an LIC to determine whether the LIC has fallen to less than 3 mg Fe/g dw.
- Use the minimum effective dose to achieve a trend of decreasing ferritin.
- Monitor LIC every 6 months.
- After 6 months of therapy, if the LIC remains greater than 7 mg Fe/g dw, increase the dose of deferasirox to a maximum of 20 mg/kg/day. Do not exceed a maximum of 20 mg/kg/day.
- If after 6 months of therapy, the LIC is 3 to 7 mg Fe/g dw, continue treatment with deferasirox at no more than 10 mg/kg/day.
- When the LIC is less than 3 mg Fe/g dw, interrupt treatment with deferasirox and continue to monitor the LIC.
- Monitor blood counts, liver function, renal function and ferritin monthly [see Warnings and Precautions (5.1, 5.2, 5.4)].
- Increase monitoring frequency for pediatric patients who have acute illness, which can cause volume depletion, such as vomiting, diarrhea, or prolonged decreased oral intake. Consider dose interruption until oral intake and volume status are normal [see Dosage and Administration (2.4, 2.5), Warnings and Precautions (5.1), Use in Specific Populations (8.4), Clinical Pharmacology (12.3)].
Restart treatment when the LIC rises again to more than 5 mg Fe/g dw.
2.3 Administration
Do not chew tablets or swallow them whole.
Take deferasirox once daily on an empty stomach at least 30 minutes before food, preferably at the same time each day. Completely disperse tablets by stirring in water, orange juice, or apple juice until a fine suspension is obtained. Disperse doses of less than 1 g in 3.5 ounces of liquid and doses of 1 g or greater in 7 ounces of liquid. After swallowing the suspension, resuspend any residue in a small volume of liquid and swallow. Do not take deferasirox with aluminum-containing antacid products [see Drug Interactions (7.1)].
2.4 Use in Patients with Baseline Hepatic or Renal Impairment
Patients with Baseline Hepatic Impairment
Mild (Child-Pugh A) Hepatic Impairment: No dose adjustment is necessary.
Moderate (Child-Pugh B) Hepatic Impairment: Reduce the starting dose by 50%.
Severe (Child-Pugh C) Hepatic Impairment: Avoid deferasirox [see Warnings and Precautions (5.2), Use in Specific Populations (8.7)].
Patients with Baseline Renal Impairment
Do not use deferasirox in adult or pediatric patients with eGFR less than 40 mL/min/1.73 m2 [see Dosage and Administration (2.5), Contraindications (4)].
For patients with renal impairment (eGFR 40 to 60 mL/min/1.73 m2), reduce the starting dose by 50% [see Use in Specific Populations (8.6)].
Exercise caution in pediatric patients with eGFR between 40 and 60 mL/min/1.73 m2. If treatment is needed, use the minimum effective dose and monitor renal function frequently. Individualize dose titration based on improvement in renal injury [see Use in Specific Populations (8.6)].
2.5 Dose Modifications for Decreases in Renal Function While on Deferasirox
Deferasirox is contraindicated in patients with eGFR less than 40 mL/min/1.73 m2 [see Contraindications (4)].
For decreases in renal function while receiving deferasirox [see Warnings and Precautions (5.1)], modify the dose as follows:
Transfusional Iron Overload
Adults:
- If the serum creatinine increases by 33% or more above the average baseline measurement, repeat the serum creatinine within 1 week, and if still elevated by 33% or more, reduce the dose by 10 mg per kg.
Pediatric Patients (ages 2 years to 17 years):
-
Reduce the dose by 10 mg/kg/day if eGFR decreases by greater than 33% below the average baseline measurement and repeat the eGFR within 1 week.
-
Interrupt deferasirox for acute illnesses, which can cause volume depletion, such as vomiting, diarrhea, or prolonged decreased oral intake, and monitor more frequently. Resume therapy as appropriate, based on assessments of renal function, when oral intake and volume status are normal. Avoid use of other nephrotoxic drugs [see Warnings and Precautions (5.1)].
-
In the setting of decreased renal function, evaluate the risk benefit profile of continued deferasirox use. Use the minimum effective deferasirox dose and monitor renal function more frequently, by evaluating tubular and glomerular function. Titrate dosing based on renal injury. Consider dose reduction or interruption and less nephrotoxic therapies until improvement of renal function. If signs of renal tubular or glomerular injury occur in the presence of other risk factors such as volume depletion, reduce or interrupt deferasirox to prevent severe and irreversible renal injury [see Warnings and Precautions (5.1)].
All Patients (regardless of age):
- Discontinue therapy for eGFR less than 40 mL/min/1.73 m2 [see Contraindications (4)].
Non-Transfusion-Dependent Thalassemia Syndromes
Adults:
- If the serum creatinine increases by 33% or more above the average baseline measurement, repeat the serum creatinine within 1 week, and if still elevated by 33% or more, interrupt therapy if the dose is 5 mg per kg, or reduce by 50% if the dose is 10 or 20 mg per kg.
Pediatric Patients (ages 10 years to 17 years):
- Reduce the dose by 5 mg/kg/day if eGFR decreases by greater than 33% below the average baseline measurement and repeat the eGFR within 1 week.
- Increase monitoring frequency for pediatric patients who have acute illnesses, which can cause volume depletion, such as vomiting, diarrhea, or prolonged decreased oral intake. Consider dose interruption until oral intake and volume status are normal. Avoid use of other nephrotoxic drugs [see Warnings and Precautions (5.1)].
- In the setting of decreased renal function, evaluate the risk benefit profile of continued deferasirox use. Use the minimum effective deferasirox dose and monitor renal function more frequently, by evaluating tubular and glomerular function. Titrate dosing based on renal injury. Consider dose reduction or interruption and less nephrotoxic therapies until improvement of renal function. If signs of renal tubular or glomerular injury occur in the presence of other risk factors such as volume depletion, reduce or interrupt deferasirox to prevent severe and irreversible renal injury [see Warnings and Precautions (5.1)].
All Patients (regardless of age):
- Discontinue therapy for eGFR less than 40 mL/min/1.73 m2 [see Contraindications (4)].
2.6 Dose Modifications Based on Concomitant Medications
UDP-glucuronosyltransferases (UGT) Inducers
Concomitant use of UGT inducers decreases deferasirox systemic exposure. Avoid the concomitant use of potent UGT inducers (e.g., rifampicin, phenytoin, phenobarbital, ritonavir) with deferasirox. If you must administer deferasirox with 1 of these agents, consider increasing the initial dose of deferasirox by 50%, and monitor serum ferritin levels and clinical responses for further dose modification [see Dosage and Administration (2.1, 2.2), Drug Interactions (7.5)].
Bile Acid Sequestrants
Concomitant use of bile acid sequestrants decreases deferasirox systemic exposure. Avoid the concomitant use of bile acid sequestrants (e.g., cholestyramine, colesevelam, colestipol) with deferasirox. If you must administer deferasirox with 1 of these agents, consider increasing the initial dose of deferasirox by 50%, and monitor serum ferritin levels and clinical responses for further dose modification [see Dosage and Administration (2.1, 2.2), Drug Interactions (7.6)].
- Transfusional Iron Overload: Initial dose for patients with estimated glomerular filtration rate (eGFR) greater than 60 mL/min/1.73 m2 is 20 mg per kg body weight once daily, as oral suspension. Calculate dose to the nearest whole tablet. (2.1)
- NTDT Syndromes: Initial dose for patients with eGFR greater than 60 mL/min/1.73 m2 is 10 mg per kg body weight once daily, as oral suspension. Calculate dose to the nearest whole tablet. (2.2)
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
Deferasirox tablets for oral suspension are available as follows:
125 mg tablets
White to off-white, round, unscored tablets, debossed with
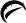 and 454
on one side and plain on the other side.
and 454
on one side and plain on the other side.
250 mg tablets
White to off-white, round, unscored tablets, debossed with
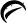 and 455
on one side and plain on the other side.
and 455
on one side and plain on the other side.
500 mg tablets
White to off-white, round, unscored tablets, debossed with
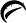 and 456
on one side and plain on the other side.
and 456
on one side and plain on the other side.
Tablets for oral suspension: 125 mg, 250 mg, 500 mg. (3)
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Dosing Instructions
Advise patients to take deferasirox tablet once daily on an empty stomach at least 30 minutes prior to food, preferably at the same time every day. Instruct patients to completely disperse the tablets in water, orange juice, or apple juice, and drink the resulting suspension immediately. After the suspension has been swallowed, resuspend any residue in a small volume of the liquid and swallow [see Dosage and Administration (2.3)].
Advise patients not to chew tablets or swallow them whole [see Dosage and Administration (2.3)].
Blood Testing
Advise patients that blood tests will be performed frequently to check for damage to kidneys, liver, or blood cells [see Warnings and Precautions (5.1, 5.2, 5.3, 5.4, 5.5)].
Acute Kidney Injury, Including Acute Renal Failure
Caution patients about the potential for kidney toxicity when taking deferasirox. Inform patients of the signs and symptoms of kidney injury. Advise patients to contact their healthcare provider immediately if they experience any of these symptoms [see Warnings and Precautions (5.1)].
Hepatic Toxicity and Failure
Caution patients about the potential for hepatic toxicity when taking deferasirox. Inform patients of the signs and symptoms of hepatic toxicity. Advise patients to contact their healthcare provider immediately if they experience any of these symptoms [see Warnings and Precautions (5.2)].
GI Ulceration and Hemorrhage
Caution patients about the potential for the development of GI ulcers or bleeding when taking deferasirox in combination with drugs that have ulcerogenic or hemorrhagic potential, such as NSAIDs, corticosteroids, oral bisphosphonates, or anticoagulants. Inform patients of the signs and symptoms of GI ulcers or bleeding. Advise patients to contact their health care provider for symptoms of heartburn but to seek immediate medical attention for symptoms of GI hemorrhage [see Warnings and Precautions (5.3)].
Allergic Reactions
Serious allergic reactions (which include swelling of the throat) have been reported in patients taking deferasirox, usually within the first month of treatment. If reactions are severe, advise patients to stop taking deferasirox immediately and seek immediate medical attention [see Warnings and Precautions (5.7)].
Severe Skin Reactions
Severe skin reactions have been reported in patients taking deferasirox. Inform patients of the signs and symptoms of severe skin reactions. If reactions are severe, advise patients to stop taking deferasirox immediately and seek immediate medical attention [see Warnings and Precautions (5.8)].
Skin Rash
Skin rashes may occur during deferasirox treatment. If the skin rash is severe, advise patients to stop taking deferasirox and seek medical attention [see Warnings and Precautions (5.9)].
Pediatric Patients with Acute Illness
Instruct pediatric patients and their caregivers to contact their healthcare provider during episodes of acute illness, especially if the patient has not been drinking fluids or the patient has volume depletion due to fever, vomiting, or diarrhea [see Warnings and Precautions (5.1)].
Auditory and Ocular Testing
Because auditory and ocular disturbances have been reported with deferasirox, conduct auditory testing and ophthalmic testing before starting deferasirox treatment and thereafter at regular intervals. Advise patients to contact their healthcare provider if they develop visual or auditory changes during treatment [see Warnings and Precautions (5.10)].
Drug Interactions
Caution patients not to take aluminum-containing antacids and deferasirox simultaneously [see Drug Interactions (7.1)].
Caution patients about potential loss of effectiveness of drugs metabolized by CYP3A4 (e.g., cyclosporine, simvastatin, hormonal contraceptive agents) when deferasirox is administered with these drugs [see Drug Interactions (7.2)].
Caution patients about potential loss of effectiveness of deferasirox when administered with drugs that are potent UGT inducers (e.g., rifampicin, phenytoin, phenobarbital, ritonavir). Based on serum ferritin levels and clinical response, consider increases in the dose of deferasirox when concomitantly used with potent UGT inducers [see Drug Interactions (7.5)].
Caution patients about potential loss of effectiveness of deferasirox when administered with drugs that are bile acid sequestrants (e.g., cholestyramine, colesevelam, colestipol). Based on serum ferritin levels and clinical response, consider increases in the dose of deferasirox when concomitantly used with bile acid sequestrants [see Drug Interactions (7.6)].
Caution patients with diabetes to monitor their glucose levels more frequently when repaglinide is used concomitantly with deferasirox [see Drug Interactions (7.3)].
Driving and Using Machines
Caution patients experiencing dizziness to avoid driving or operating machinery [see Adverse Reactions (6.1)].
Dispense with Medication Guide available at: www.tevausa.com/medguides
Manufactured For:
Teva Pharmaceuticals
****Parsippany, NJ 07054
Rev. J 8/2021
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no studies with the use of deferasirox in pregnant women to inform drug-associated risks.
Administration of deferasirox to rats during pregnancy resulted in decreased offspring viability and an increase in renal anomalies in male offspring at doses that were about or less than the recommended human dose on an mg/m2 basis. No fetal effects were noted in pregnant rabbits at doses equivalent to the human recommended dose on an mg/m2 basis. Deferasirox should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. However, the background risk in the U.S. general population of major birth defects is 2% to 4% and of miscarriage is 15% to 20% of clinically recognized pregnancies.
Data
Animal Data
In embryo-fetal developmental studies, pregnant rats and rabbits received oral deferasirox during the period of organogenesis at doses up to 100 mg/kg/day in rats and 50 mg/kg/day in rabbits (1.2 times the maximum recommended human dose (MRHD) on an mg/m2 basis). These doses resulted in maternal toxicity but no fetal harm was observed.
In a prenatal and postnatal developmental study, pregnant rats received oral deferasirox daily from organogenesis through lactation day 20 at doses of 10, 30, and 90 mg/kg/day (0.1, 0.3, and 1.0 times the MRHD on an mg/m2 basis). Maternal toxicity, loss of litters, and decreased offspring viability occurred at 90 mg/kg/day (1.0 times the MRHD on a mg/m2 basis) and increases in renal anomalies in male offspring occurred at 30 mg/kg/day (0.3 times the MRHD on a mg/m2 basis).
8.2 Lactation
Risk Summary
No data are available regarding the presence of deferasirox or its metabolites in human milk, the effects of the drug on the breastfed child, or the effects of the drug on milk production. Deferasirox and its metabolites were excreted in rat milk. Because many drugs are excreted in human milk, and because of the potential for serious adverse reactions in a breastfeeding child from deferasirox and its metabolites, a decision should be made whether to discontinue breastfeeding or to discontinue the drug, taking into account the importance of the drug to the mother.
8.3 Females and Males of Reproductive Potential
Contraception
Counsel patients to use non-hormonal method(s) of contraception since deferasirox can render hormonal contraceptives ineffective [see Drug Interactions (7.2)].
8.4 Pediatric Use
Transfusional Iron Overload
The safety and effectiveness of deferasirox have been established in pediatric patients 2 years of age and older for the treatment of transfusional iron overload [see Dosage and Administration (2.1)].
Safety and effectiveness have not been established in pediatric patients less than 2 years of age for the treatment of transfusional iron overload.
Pediatric approval for treatment of transfusional iron overload was based on clinical studies of 292 pediatric patients 2 years to less than 16 years of age with various congenital and acquired anemias. Seventy percent of these patients had beta-thalassemia [see Indications and Usage (1), Dosage and Administration (2.1), Clinical Studies (14)]. In those clinical studies, 173 children (ages 2 to < 12 years) and 119 adolescents (ages 12 to < 17 years) were exposed to deferasirox.
Iron Overload in Non-Transfusion-Dependent Thalassemia Syndromes
The safety and effectiveness of deferasirox have been established in patients 10 years of age and older for the treatment of chronic iron overload with non- transfusion-dependent thalassemia (NTDT) syndromes [see Dosage and Administration (2.2)].
Safety and effectiveness have not been established in patients less than 10 years of age with chronic iron overload in NTDT syndromes.
Pediatric approval for treatment of NTDT syndromes with liver iron (Fe) concentration (LIC) of at least 5 mg Fe per gram of dry weight and a serum ferritin greater than 300 mcg/L was based on 16 pediatric patients treated with deferasirox therapy (10 years to less than 16 years of age) with chronic iron overload and NTDT. Use of deferasirox in these age groups is supported by evidence from adequate and well-controlled studies of deferasirox in adult and pediatric patients [see Indications and Usage (1.2), Dosage and Administration (2.2), Clinical Studies (14)].
In general, risk factors for deferasirox-associated kidney injury include preexisting renal disease, volume depletion, overchelation, and concomitant use of other nephrotoxic drugs. Acute kidney injury, and acute liver injury and failure has occurred in pediatric patients. In a pooled safety analysis, pediatric patients with higher deferasirox exposures had a greater probability of renal toxicity and decreased renal function, resulting in increased deferasirox exposure and progressive renal toxicity/kidney injury. Higher rates of renal adverse reactions have been identified among pediatric patients receiving deferasirox doses greater than 25 mg/kg/day when their serum ferritin values were less than 1,000 mcg/L [see Dosage and Administration (2.5), Warnings and Precautions (5.1, 5.6), Adverse Reactions (6.1, 6.2)].
Monitoring Recommendations for pediatric patients with Transfusional Iron Overload and NTDT It is recommended that serum ferritin be monitored every month to assess the patient’s response to therapy and to minimize the risk of overchelation [see Warnings and Precautions (5.6)].
Monitor renal function by estimating GFR using an eGFR prediction equation appropriate for pediatric patients and evaluate renal tubular function. Monitor renal function more frequently in pediatric patients in the presence of renal toxicity risk factors, including episodes of dehydration, fever and acute illness that may result in volume depletion or decreased renal perfusion. Use the minimum effective dose [see Warnings and Precautions (5.1)].
Interrupt deferasirox in pediatric patients with transfusional iron overload for acute illnesses, which can cause volume depletion, such as vomiting, diarrhea, or prolonged decreased oral intake, and monitor more frequently. Resume therapy as appropriate, based on assessments of renal function, when oral intake and volume status are normal. Evaluate the risk benefit profile of continued deferasirox use in the setting of decreased renal function. Avoid use of other nephrotoxic drugs [see Dosage and Administration (2.5), Warnings and Precautions (5.1)].
Juvenile Animal Toxicity Data
Renal toxicity was observed in adult mice, rats, and marmoset monkeys administered deferasirox at therapeutic doses. In a neonatal and juvenile toxicity study in rats, deferasirox was administered orally from postpartum Day 7 through 70, which equates to a human age range of term neonate through adolescence. Increased renal toxicity was identified in juvenile rats compared to adult rats at a dose based on mg/m2 approximately 0.4 times the recommended dose of 20 mg/kg/day. A higher frequency of renal abnormalities was noted when deferasirox was administered to non-iron overloaded animals compared to iron overloaded animals.
Additional pediatric use information is approved for Novartis Pharmaceuticals Corporation’s EXJADE® (deferasirox) tablets for oral suspension. However, due to Novartis Pharmaceuticals Corporation’s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
8.5 Geriatric Use
Four hundred thirty-one (431) patients greater than or equal to 65 years of age were studied in clinical trials of deferasirox in the transfusional iron overload setting. Two hundred twenty-five (225) of these patients were between 65 and 75 years of age while 206 were greater than or equal to 75 years of age. The majority of these patients had myelodysplastic syndrome (MDS) (n = 393). In these trials, elderly patients experienced a higher frequency of adverse reactions than younger patients. Monitor elderly patients for early signs or symptoms of adverse reactions that may require a dose adjustment. Elderly patients are at increased risk for toxicity due to the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. Dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range.
In elderly patients, including those with MDS, individualize the decision to remove accumulated iron based on clinical circumstances and the anticipated clinical benefit and risks of deferasirox therapy.
8.6 Renal Impairment
Deferasirox is contraindicated in patients with eGFR less than 40 mL/min/1.73 m2 [see Contraindications (4)]. For patients with renal impairment (eGFR 40 to 60 mL/min/1.73 m2), reduce the starting dose by 50% [see Dosage and Administration (2.4)]. Exercise caution in pediatric patients with eGFR between 40 and 60 mL/min/1.73 m2 [see Dosage and Administration (2.4)]. If treatment is needed, use the minimum effective dose with enhanced monitoring of glomerular and renal tubular function. Individualize dose titration based on improvement in renal injury [see Dosage and Administration (2.4, 2.5)].
Deferasirox can cause glomerular dysfunction, renal tubular toxicity, or both, and can result in acute renal failure. Monitor all patients closely for changes in eGFR and renal tubular dysfunction during deferasirox treatment. If either develops, consider dose reduction, interruption or discontinuation of deferasirox until glomerular or renal tubular function returns to baseline [see Dosage and Administration (2.4, 2.5), Warnings and Precautions (5.1)].
8.7 Hepatic Impairment
Avoid the use of deferasirox in patients with severe (Child-Pugh C) hepatic impairment. For patients with moderate (Child-Pugh B) hepatic impairment, the starting dose should be reduced by 50%. Closely monitor patients with mild (Child-Pugh A) or moderate (Child-Pugh B) hepatic impairment for efficacy and adverse reactions that may require dose titration [see Dosage and Administration (2.4), Warnings and Precautions (5.2)].
- Lactation: Advise women not to breastfeed. (8.2)
OVERDOSAGE SECTION
10 OVERDOSAGE
Cases of overdose (2 to 3 times the prescribed dose for several weeks) have been reported. In one case, this resulted in hepatitis, which resolved without long-term consequences after a dose interruption. In one pediatric case, a dose of 2 to 3 times the prescribed dose for 6 days, resulted in acute renal failure requiring hemofiltration and acute liver injury/failure, which were reversible with intensive care support. Single doses up to 80 mg per kg per day in iron overloaded beta-thalassemic patients have been tolerated with nausea and diarrhea noted. In healthy volunteers, single doses of up to 40 mg per kg per day were tolerated.
Early signs of acute overdose are digestive effects such as abdominal pain, diarrhea, nausea, and vomiting. Hepatic and renal disorders have been reported, including cases of liver enzyme and creatinine increased with recovery after treatment discontinuation. An erroneously administered single dose of 90 mg/kg led to Fanconi syndrome which resolved after treatment.
There is no specific antidote for deferasirox. In case of overdose, it may be treated with induction of vomiting or gastric lavage, and by symptomatic treatment.
DESCRIPTION SECTION
11 DESCRIPTION
Deferasirox is an iron chelating agent. Deferasirox Tablets For Oral Suspension contain 125 mg, 250 mg, or 500 mg of deferasirox. Deferasirox is designated chemically as 4-[3,5-Bis (2-hydroxyphenyl)-1H-1,2,4-triazol-1-yl]-benzoic acid and its structural formula is:
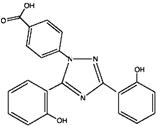
C21H15N3O4 M.W. 373.4
Deferasirox is an off-white to pale yellow powder.
Inactive Ingredients: colloidal silicon dioxide, crospovidone, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone and sodium lauryl sulfate.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Deferasirox is an orally active chelator that is selective for iron (as Fe3+). It is a tridentate ligand that binds iron with high affinity in a 2:1 ratio. Although deferasirox has very low affinity for zinc and copper there are variable decreases in the serum concentration of these trace metals after the administration of deferasirox. The clinical significance of these decreases is uncertain.
12.2 Pharmacodynamics
Pharmacodynamic effects tested in an iron balance metabolic study showed that deferasirox (10, 20, and 40 mg per kg per day) was able to induce a mean net iron excretion (0.119, 0.329, and 0.445 mg Fe/kg body weight per day, respectively) within the clinically relevant range (0.1 to 0.5 mg per kg per day). Iron excretion was predominantly fecal.
An analysis of pooled pediatric clinical trial data found a statistically significant relationship between exposure and the probability of renal toxicity (increase in serum creatinine and urinary protein), resulting in a decrease in renal function. Decreases in renal function resulted in an increase in deferasirox exposure, which may increase the probability of renal toxicity.
Cardiac Electrophysiology
At the maximum approved recommended dose, deferasirox does not prolong the QT interval to any clinically relevant extent.
12.3 Pharmacokinetics
Absorption
Deferasirox is absorbed following oral administration with median times to maximum plasma concentration (Tmax) of about 1.5 to 4 hours. The Cmax and area under the curve (AUC) of deferasirox increase approximately linearly with dose after both single administration and under steady-state conditions. Exposure to deferasirox increased by an accumulation factor of 1.3 to 2.3 after multiple doses. The absolute bioavailability (AUC) of deferasirox tablets for oral suspension is 70% compared to an intravenous dose. The bioavailability (AUC) of deferasirox was variably increased when taken with a meal.
Distribution
Deferasirox is highly (~99%) protein bound almost exclusively to serum albumin. The percentage of deferasirox confined to the blood cells was 5% in humans. The volume of distribution at steady state (Vss) of deferasirox is 14.37 ± 2.69 L in adults.
Metabolism
Glucuronidation is the main metabolic pathway for deferasirox, with subsequent biliary excretion. Deconjugation of glucuronidates in the intestine and subsequent reabsorption (enterohepatic recycling) is likely to occur. Deferasirox is mainly glucuronidated by UGT1A1 and to a lesser extent UGT1A3. CYP450-catalyzed (oxidative) metabolism of deferasirox appears to be minor in humans (about 8%). Deconjugation of glucuronide metabolites in the intestine and subsequent reabsorption (enterohepatic recycling) was confirmed in a healthy volunteer study in which the administration of cholestyramine 12 g twice daily (strongly binds to deferasirox and its conjugates) 4 and 10 hours after a single dose of deferasirox resulted in a 45% decrease in deferasirox exposure (AUC) by interfering with the enterohepatic recycling of deferasirox.
Excretion
Deferasirox and metabolites are primarily (84% of the dose) excreted in the feces. Renal excretion of deferasirox and metabolites is minimal (8% of the administered dose). The mean elimination half-life (t1/2) ranged from 8 to 16 hours following oral administration.
Drug Interactions
Midazolam: In healthy volunteers, the concomitant administration of deferasirox and midazolam (a CYP3A4 probe substrate) resulted in a decrease of midazolam peak concentration by 23% and exposure by 17%. In the clinical setting, this effect may be more pronounced. The study was not adequately designed to conclusively assess the potential induction of CYP3A4 by deferasirox [see Drug Interactions (7.2)].
Repaglinide: In a healthy volunteer study, the concomitant administration of deferasirox (30 mg per kg/day for 4 days) and the CYP2C8 probe substrate repaglinide (single dose of 0.5 mg) resulted in an increase in repaglinide systemic exposure (AUC) to 2.3-fold of control and an increase in Cmax of 62% [see Drug Interactions (7.3)].
Theophylline: In a healthy volunteer study, the concomitant administration of deferasirox (repeated dose of 30 mg per kg/day) and the CYP1A2 substrate theophylline (single dose of 120 mg) resulted in an approximate doubling of the theophylline AUC and elimination half-life. The single dose Cmax was not affected, but an increase in theophylline Cmax is expected to occur with chronic dosing [see Drug Interactions (7.4)].
Rifampicin: In a healthy volunteer study, the concomitant administration of deferasirox (single dose of 30 mg per kg) and the potent UDP- glucuronosyltransferase (UGT) inducer rifampicin (600 mg/day for 9 days) resulted in a decrease of deferasirox systemic exposure (AUC) by 44% [see Drug Interactions (7.5)].
Cholestyramine: The concomitant use of deferasirox with bile acid sequestrants may result in a decrease in deferasirox efficacy. In healthy volunteers, the administration of cholestyramine after a single dose of deferasirox resulted in a 45% decrease in deferasirox exposure (AUC) [see Drug Interactions (7.6)].
Busulfan: Concomitant administration of deferasirox and busulfan resulted in an increase of busulfan exposure (AUC).
In vitro Studies:
- Cytochrome P450 Enzymes: Deferasirox inhibits human CYP3A4, CYP2C8, CYP1A2, CYP2A6, CYP2D6, and CYP2C19 in vitro.
- Transporter Systems: The addition of cyclosporin A (PgP/MRP1/MRP2 inhibitor) or verapamil (PgP/MRP1 inhibitor) did not influence ICL670 permeability in vitro.
Pharmacokinetics in Specific Populations
Pediatric: Following oral administration of single or multiple doses, systemic exposure of adolescents and children to deferasirox was less than in adult patients. In children less than 6 years of age, systemic exposure was about 50% lower than in adults.
Geriatric: The pharmacokinetics of deferasirox have not been studied in elderly patients (65 years of age or older).
Gender: Females have a moderately lower apparent clearance (by 17.5%) for deferasirox compared to males.
Renal Impairment: Compared to patients with MDS and eGFR greater than 60 mL/min/1.73 m2, patients with MDS and eGFR 40 to 60 mL/min/1.73 m2 (n = 34) had approximately 50% higher mean deferasirox trough plasma concentrations.
Hepatic Impairment: In a single dose (20 mg/kg) study in patients with varying degrees of hepatic impairment, deferasirox exposure was increased compared to patients with normal hepatic function. The average total (free and bound) AUC of deferasirox increased 16% in 6 patients with mild (Child-Pugh A) hepatic impairment, and 76% in 6 patients with moderate (Child-Pugh B) hepatic impairment compared to 6 patients with normal hepatic function. The impact of severe (Child-Pugh C) hepatic impairment was assessed in only 1 patient.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
A 104-week oral carcinogenicity study in Wistar rats showed no evidence of carcinogenicity from deferasirox at doses up to 60 mg per kg per day (0.48 times the MRHD on an mg/m2 basis). A 26-week oral carcinogenicity study in p53 (+/-) transgenic mice has shown no evidence of carcinogenicity from deferasirox at doses up to 200 mg per kg per day (0.81 times the MRHD on a mg/m2 basis) in males and 300 mg per kg per day (1.21 times the MRHD on a mg/m2 basis) in females.
Deferasirox was negative in the Ames test and chromosome aberration test with human peripheral blood lymphocytes. It was positive in 1 of 3 in vivo oral rat micronucleus tests.
Deferasirox at oral doses up to 75 mg per kg per day (0.6 times the MRHD on a mg/m2 basis) was found to have no adverse effect on fertility and reproductive performance of male and female rats.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
Transfusional Iron Overload
The primary efficacy study, Study 1 (NCT00061750), was a multicenter, open- label, randomized, active-comparator control study to compare deferasirox and deferoxamine in patients with beta-thalassemia and transfusional hemosiderosis. Patients greater than or equal to 2 years of age were randomized in a 1:1 ratio to receive either oral deferasirox at starting doses of 5, 10, 20, or 30 mg per kg once daily or subcutaneous deferoxamine at starting doses of 20 to 60 mg per kg for at least 5 days per week based on LIC at baseline (2 to 3, greater than 3 to 7, greater than 7 to 14, and greater than 14 mg Fe/g dry weight). Patients randomized to deferoxamine who had LIC values less than 7 mg Fe/g dry weight were permitted to continue on their prior deferoxamine dose, even though the dose may have been higher than specified in the protocol.
Patients were to have a liver biopsy at baseline and end of study (after 12 months) for LIC. The primary efficacy endpoint was defined as a reduction in LIC of greater than or equal to 3 mg Fe/g dry weight for baseline values greater than or equal to 10 mg Fe/g dry weight, reduction of baseline values between 7 and less than 10 to less than 7 mg Fe/g dry weight, or maintenance or reduction for baseline values less than 7 mg Fe/g dry weight.
A total of 586 patients were randomized and treated, 296 with deferasirox and 290 with deferoxamine. The mean age was 17.1 years (range, 2 to 53 years); 52% were females and 88% were Caucasian. The primary efficacy population consisted of 553 patients (deferasirox n = 276; deferoxamine n = 277) who had LIC evaluated at baseline and 12 months or discontinued due to an adverse reaction. The percentage of patients achieving the primary endpoint was 52.9% for deferasirox and 66.4% for deferoxamine. The relative efficacy of deferasirox to deferoxamine cannot be determined from this study.
In patients who had an LIC at baseline and at end of study, the mean change in LIC was -2.4 mg Fe/g dry weight in patients treated with deferasirox and -2.9 mg Fe/g dry weight in patients treated with deferoxamine.
Reduction of LIC and serum ferritin was observed with deferasirox doses of 20 to 30 mg per kg per day. Deferasirox doses below 20 mg per kg per day failed to provide consistent lowering of LIC and serum ferritin levels (Figure 1). Therefore, a starting dose of 20 mg per kg per day is recommended [see Dosage and Administration (2.1)].
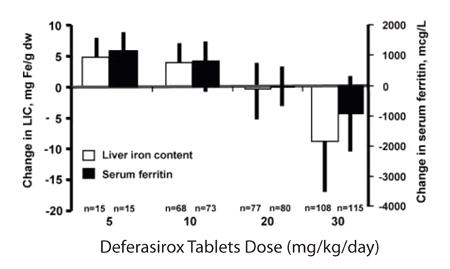
Figure 1. Changes in Liver Iron Concentration and Serum Ferritin Following Deferasirox (5 to 30 mg per kg per day) in Study 1
Study 2 (NCT00061763) was an open-label, noncomparative trial of efficacy and safety of deferasirox given for 1 year to patients with chronic anemias and transfusional hemosiderosis. Similar to Study 1, patients received 5, 10, 20, or 30 mg per kg per day of deferasirox based on baseline LIC.
A total of 184 patients were treated in this study: 85 patients with beta- thalassemia and 99 patients with other congenital or acquired anemias (myelodysplastic syndromes, n = 47; Diamond-Blackfan syndrome, n = 30; other, n = 22). Nineteen percent (19%) of patients were less than 16 years of age and 16% were greater than 65 years of age. There was a reduction in the absolute LIC from baseline to end of study (-4.2 mg Fe/g dry weight).
Study 3 (NCT00067080) was a multicenter, open-label, randomized trial of the safety and efficacy of deferasirox relative to deferoxamine given for 1 year in patients with sickle cell disease and transfusional hemosiderosis. Patients were randomized to deferasirox at doses of 5, 10, 20, or 30 mg per kg per day or subcutaneous deferoxamine at doses of 20 to 60 mg per kg per day for 5 days per week according to baseline LIC.
A total of 195 patients were treated in this study: 132 with deferasirox and 63 with deferoxamine. Forty-four percent (44%) of patients were less than 16 years of age and 91% were black. At end of study, the mean change in LIC (as measured by magnetic susceptometry by a superconducting quantum interference device) in the per protocol-1 (PP-1) population, which consisted of patients who had at least 1 post-baseline LIC assessment, was -1.3 mg Fe/g dry weight for patients receiving deferasirox (n = 113) and -0.7 mg Fe/g dry weight for patients receiving deferoxamine (n = 54).
One-hundred five (105) patients with thalassemia major and cardiac iron overload were enrolled in a study assessing the change in cardiac magnetic resonance imaging (MRI) T2* value (measured in milliseconds, ms) before and after treatment with deferasirox. Cardiac T2* values at baseline ranged from 5 to less than 20 ms. The geometric mean of cardiac T2* in the 68 patients who completed 3 years of deferasirox therapy increased from 11.98 ms at baseline to 17.12 ms at 3 years. Cardiac T2* values improved in patients with severe cardiac iron overload (less than 10 ms) and in those with mild to moderate cardiac iron overload (greater than or equal to 10 to less than 20 ms). The clinical significance of these observations is unknown.
Six hundred twenty-seven (627) patients with MDS were enrolled across 5 uncontrolled trials. Two hundred thirty-nine (239) of the 627 patients were enrolled in trials that limited enrollment to patients with IPSS Low or Intermediate 1 risk MDS, and the remaining 388 patients were enrolled in trials that did not specify MDS risk stratification but required a life expectancy of greater than 1 year. Planned duration of treatment in these trials ranged from 1 year (365 patients) to 5 years (47 patients). These trials evaluated the effects of deferasirox therapy on parameters of iron overload, including LIC (125 patients) and serum ferritin (627 patients). The percent of patients completing planned duration of treatment was 51% in the largest 1-year study, 52% in the 3-year study and 22% in the 5-year study. The major causes for treatment discontinuation were withdrawal of consent, adverse reaction, and death. Over 1 year of follow-up across these pooled studies, mean change in serum ferritin was -332.8 (± 2615.59) mcg/L (n = 593) and mean change in LIC was -5.9 (± 8.32) mg Fe/g dw (n = 68). Results of these pooled studies in 627 patients with MDS suggest a progressive decrease in serum ferritin and LIC beyond 1 year in those patients who are able to continue deferasirox.
Non-Transfusion Dependent Thalassemia
Study 5 (NCT00873041) was a randomized, double-blind, placebo-controlled trial of treatment with deferasirox for patients 10 years of age or older with NTDT syndromes and iron overload. Eligible patients had an LIC of at least 5 mg Fe/g dw measured by R2 MRI and a serum ferritin exceeding 300 mcg/L at screening (2 consecutive values at least 14 days apart from each other). A total of 166 patients were randomized, 55 to the deferasirox 5 mg/kg/day dose group, 55 to the deferasirox 10 mg/kg/day dose group, and 56 to placebo (28 to each matching placebo group). Doses could be increased after 6 months if the LIC exceeded 7 mg Fe/g dw and the LIC reduction from baseline was less than 15%. The patients enrolled included 89 males and 77 females. The underlying disease was beta-thalassemia intermedia in 95 (57%) patients, HbE beta- thalassemia in 49 (30%) patients, and alpha-thalassemia in 22 (13%) patients. There were 17 pediatric patients in the study. Caucasians comprised 57% of the study population and Asians comprised 42%. The median baseline LIC (range) for all patients was 12.1 (2.6 to 49.1) mg Fe/g dw. Follow-up was for 1 year. The primary efficacy endpoint of change in LIC from baseline to Week 52 was statistically significant in favor of both deferasirox dose groups compared with placebo (p ≤ 0.001) (Table 5). Furthermore, a statistically significant dose effect of deferasirox was observed in favor of the 10 mg/kg/day dose group (10 versus 5 mg/kg/day, p = 0.009). In a descriptive analysis, the target LIC (less than 5 mg Fe/g dw) was reached by 15 (27%) of 55 patients in the 10 mg/kg/day arm, 8 (15%) of 55 patients in the 5 mg/kg/day arm and 2 (4%) of 56 patients in the combined placebo groups.
Study 6 (NCT00873041) was an open-label trial of deferasirox for the treatment of patients previously enrolled on Study 5, including cross-over to active treatment for those previously treated with placebo. The starting dose of deferasirox in Study 6 was assigned based on the patient’s LIC at completion of Study 5, being 20 mg/kg/day for an LIC exceeding 15 mg Fe/g dw, 10 mg/kg/day for LIC 3 to 15 mg Fe/g dw, and observation if the LIC was less than 3 mg Fe/g dw. Patients could continue on 5 mg/kg/day if they had previously exhibited at least a 30% reduction in LIC. Doses could be increased to a maximum of 20 mg/kg/day after 6 months if the LIC was more than 7 mg Fe/g dw and the LIC reduction from baseline was less than 15%. The primary efficacy endpoint in Study 6 was the proportion of patients achieving an LIC less than 5 mg Fe/g dw. A total of 133 patients were enrolled. Twenty patients began Study 6 with an LIC less than 5 mg Fe/g dw. Of the 113 patients with a baseline LIC of at least 5 mg Fe/g dw in Study 6, the target LIC (less than 5 mg Fe/g dw) was reached by 39 patients (35%). The responders included 4 (10%) of 39 patients treated at 20 mg/kg/day for a baseline LIC exceeding 15 mg Fe/g dw, and 31 (51%) of 61 patients treated at 10 mg/kg/day for a baseline LIC between 5 and 15 mg Fe/g dw. The absolute change in LIC at Week 52 by starting dose is shown in Table 5 below.
Table 5. Absolute Change in LIC at Week 52 in Patients with NTDT|
Deferasirox Tablets for Oral SuspensionStarting Dosea | ||||
|
Placebo |
5 mg/kg/day |
10 mg/kg/day |
20 mg/kg/day | |
|
Study 5****b | ||||
|
Number of Patients |
n = 54 |
n = 51 |
n = 54 |
|
|
Mean LIC at Baseline (mg Fe/g dw) |
16.1 |
13.4 |
14.4 |
|
|
Mean Change (mg Fe/g dw) |
+0.4 |
-2.0 |
-3.8 |
|
|
(95% Confidence Interval) |
(-0.6, +1.3) |
(-2.9, -1.0) |
(-4.8, -2.9) |
|
|
Study 6 | ||||
|
Number of Patients |
|
n = 8 |
n = 77 |
n = 43 |
|
Mean LIC at Baseline (mg Fe/g dw) |
|
5.6 |
8.8 |
23.5 |
|
Mean Change (mg Fe/g dw) |
|
-1.5 |
-2.8 |
-9.1 |
|
(95% Confidence Interval) |
|
(-3.7, +0.7) |
(-3.4, -2.2) |
(-11.0, -7.3) |
|
Abbreviation: LIC, liver iron concentration; NTDT, non-transfusion-dependent thalassemia. | ||||
|
aRandomized dose in Study 5 or assigned starting dose in Study 6. | ||||
|
bLeast square mean change for Study 5. |
Additional pediatric use information is approved for Novartis Pharmaceuticals Corporation’s EXJADE® (deferasirox) tablets for oral suspension. However, due to Novartis Pharmaceuticals Corporation’s marketing exclusivity rights, this drug product is not labeled with that information.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Deferasirox is provided as 125 mg, 250 mg, and 500 mg Tablets For Oral Suspension.
125 mg – White to off-white, round, unscored tablets, debossed with
 and 454
on one side and plain on the other side. Tablets are supplied in bottles of 30
(NDC 45963-454-30) with a child-resistant closure.
and 454
on one side and plain on the other side. Tablets are supplied in bottles of 30
(NDC 45963-454-30) with a child-resistant closure.
250 mg – White to off-white, round, unscored tablets, debossed with
 and 455
on one side and plain on the other side. Tablets are supplied in bottles of 30
(NDC 45963-455-30) with a child-resistant closure.
and 455
on one side and plain on the other side. Tablets are supplied in bottles of 30
(NDC 45963-455-30) with a child-resistant closure.
500 mg – White to off-white, round, unscored tablets, debossed with
 and 456
on one side and plain on the other side. Tablets are supplied in bottles of 30
(NDC 45963-456-30) with a child-resistant closure.
and 456
on one side and plain on the other side. Tablets are supplied in bottles of 30
(NDC 45963-456-30) with a child-resistant closure.
Store at 25°C (77°F); excursions are permitted to 15 to 30°C (59 to 86°F) [see USP Controlled Room Temperature]. Protect from moisture.
Dispense in a tight, light-resistant container as defined in the USP.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
SPL MEDGUIDE SECTION
MEDICATION GUIDE
|
Dispense with Medication Guide available at: www.tevausa.com/medguides | |
|
Deferasirox (dee fer' a sir ox) T****ablets For Oral Suspension | |
|
What is the most important information I should know about deferasirox? Deferasirox can cause serious side effects, including: Kidney problems. Deferasirox can cause sudden (acute) kidney problems, including kidney failure that may require treatment with dialysis, and may cause death. Deaths have happened mostly in people who also have other health problems and had a blood disorder that was in an advanced stage. Adults and children who already have kidney problems and are taking certain medicines with deferasirox may also have an increased risk of sudden kidney problems. Be sure to tell your healthcare provider about all the medicines you take during treatment with deferasirox. Your healthcare provider should do blood and urine tests to check your or your child’s kidney function before and during treatment with deferasirox.Call your or your child’s healthcare provider right away if: *your child becomes sick with fever, vomiting, or diarrhea and cannot drink fluids normally during treatment with deferasirox. Your child may be dehydrated. Your child’s healthcare provider may need to temporarily stop treatment with deferasirox and treat your child for dehydration to help prevent kidney problems. Your child’s healthcare provider may monitor your child’s kidney function more closely. *you notice that you or your child are passing less urine than usual during treatment with deferasirox. Liver problems. Deferasirox can cause liver problems, including liver failure that can sometimes cause death. Liver problems with deferasirox may be more common in people who are over 55 years of age but can also happen in children. Liver failure has happened more often in people with cirrhosis of the liver and failure of other organs. Liver failure has also happened along with kidney problems in certain children who become dehydrated.See “Kidney problems” above. Your healthcare provider should do blood tests to check your liver function before you start and regularly during treatment with deferasirox.Call your healthcare provider right away, if you develop any of the following signs and symptoms: | |
|
|
|
|
|
Bleeding, ulcers, and tears of the stomach or intestine. Severe stomach and intestine bleeding (hemorrhage) that have caused death have happened in some people treated with deferasirox, especially in elderly people who have advanced blood cancers or low platelet counts. Some people have also had ulcers of the stomach or intestine, sometimes with tears (perforation) that have caused death. In some people who have taken deferasirox, including children and adolescents, irritation of the upper gastrointestinal tract, ulcers, and bleeding have happened, but did not cause death. Your risk of severe bleeding (hemorrhage) may be increased if you take deferasirox along with other medicines that can cause ulcers or bleeding, such as: | |
|
|
|
|
|
Before you start taking deferasirox, tell your healthcare provider if you are taking one of these medicines. Ask your healthcare provider if you are not sure. If you develop an ulcer of the stomach or intestine, or severe bleeding, your healthcare provider may stop deferasirox. Elderly people may be at a higher risk of developing serious side effects and death due to serious side effects with deferasirox. Your healthcare provider may need to monitor you more closely during treatment with deferasirox.
See “What are the possible side effects of deferasirox?” for more information about side effects. | |
|
What is****deferasirox? Deferasirox is a prescription medicine that is used to treat:
It is not known if deferasirox is safe and effective when used with other medicines to treat an increased amount of iron in the blood. It is not known if deferasirox is safe and effective for treating children under 2 years of age who have an increased amount of iron in their blood for a long period of time (chronic) caused by repeated blood transfusions. | |
|
Do not takedeferasiroxi****f you:
Ask your healthcare provider if you are not sure if you have any of the medical conditions listed above. | |
|
Before takingdeferasirox, tell your healthcare provider about all of your medical conditions, including if you:
**Tell your healthcare provider about all the medicines you take, **including prescription medicines, over-the-counter medicines, vitamins, and herbal supplements. Some medicines may affect how deferasirox works, and deferasirox may affect how other medicines work. Also,your risk of sudden kidney problems or severe bleeding may be increased if you take deferasiroxwith certain medicines. See “What is the most important information I should know aboutdeferasirox?”
Ask your healthcare provider if you are not sure if you take one of these medicines. ** Know the medicines you take. Keep a list of them to show your healthcare provider or pharmacist when you get a new medicine.** | |
|
How should I takedeferasirox?
| |
|
What should I avoid while takingdeferasirox**?**
| |
|
What are the possible side effects ofdeferasirox**?** Deferasiroxca****n cause serious side effects, including: Se**e “What is the most important information I should know about*deferasirox**?”** *E**ffects on your bone marrow.**Deferasirox can affect your bone marrow and cause you to have a low white blood cell count which can be serious, decreased platelets, or worsening of your anemia, and may lead to death. Your risk for effects on your bone marrow may be increased if you already have other blood disorders. Your healthcare provider will do blood tests to monitor your blood cell counts for these problems. ***Serious allergic reactions.**Deferasirox may cause serious allergic reactions, which usually start within the first month of treatment.Get medical help right away if you develop any of the following symptoms of a serious allergic reaction including: | |
|
|
|
|
|
|
| |
|
*Ski**n rash and severe skin reactions.**Skin rashes are common with deferasirox. If you get a more severe rash, your healthcare provider may temporarily stop deferasirox. Severe skin reactions can also happen withdeferasirox and can be life-threatening or lead to death. Get medical help right away if you develop any one or more of the following signs and symptoms of a severe skin reaction, including: | |
|
|
|
|
|
|
|
***Hearing and vision problems.**Deferasirox can cause decreased hearing and changes in your vision, including cataracts, increased pressure in your eye, and problems with your retinas. Your healthcare provider should do hearing and vision tests before you start and then regularly during treatment. Your healthcare provider may decrease your dose or stop deferasirox if you develop hearing or vision problems. Tell your healthcare provider if you develop any changes in your vision or hearing during treatment with deferasirox. The most common side effects in anyone who takes deferasirox include: diarrhea and nausea. Other common side effects in people with too much iron in their blood due to repeated blood transfusions include: vomiting, stomach-area (abdomen) pain, and an abnormal kidney function blood test. These are not all the possible side effects of deferasirox. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. | |
|
How should I store deferasirox**?**
Keep deferasirox and all medicines out of the reach of children. | |
|
General information about the safe and effective use of****deferasirox. Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use deferasirox for a condition for which it was not prescribed. Do not give deferasirox to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about deferasirox that is written for health professionals. | |
|
What are the ingredients indeferasirox**?** Active ingredient: deferasirox Inactive ingredients: Colloidal silicon dioxide, crospovidone, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone and sodium lauryl sulfate. Additional pediatric use information is approved for Novartis Pharmaceuticals Corporation’s EXJADE® (deferasirox) tablets for oral suspension. However, due to Novartis Pharmaceuticals Corporation’s marketing exclusivity rights, this drug product is not labeled with that pediatric information. Manufactured For:** Teva Pharmaceuticals,**Parsippany, NJ 07054 For more information, call 1-888-838-2872 | |
|
This Medication Guide has been approved by the U.S. Food and Drug Administration. Rev. E 8/2021 |
