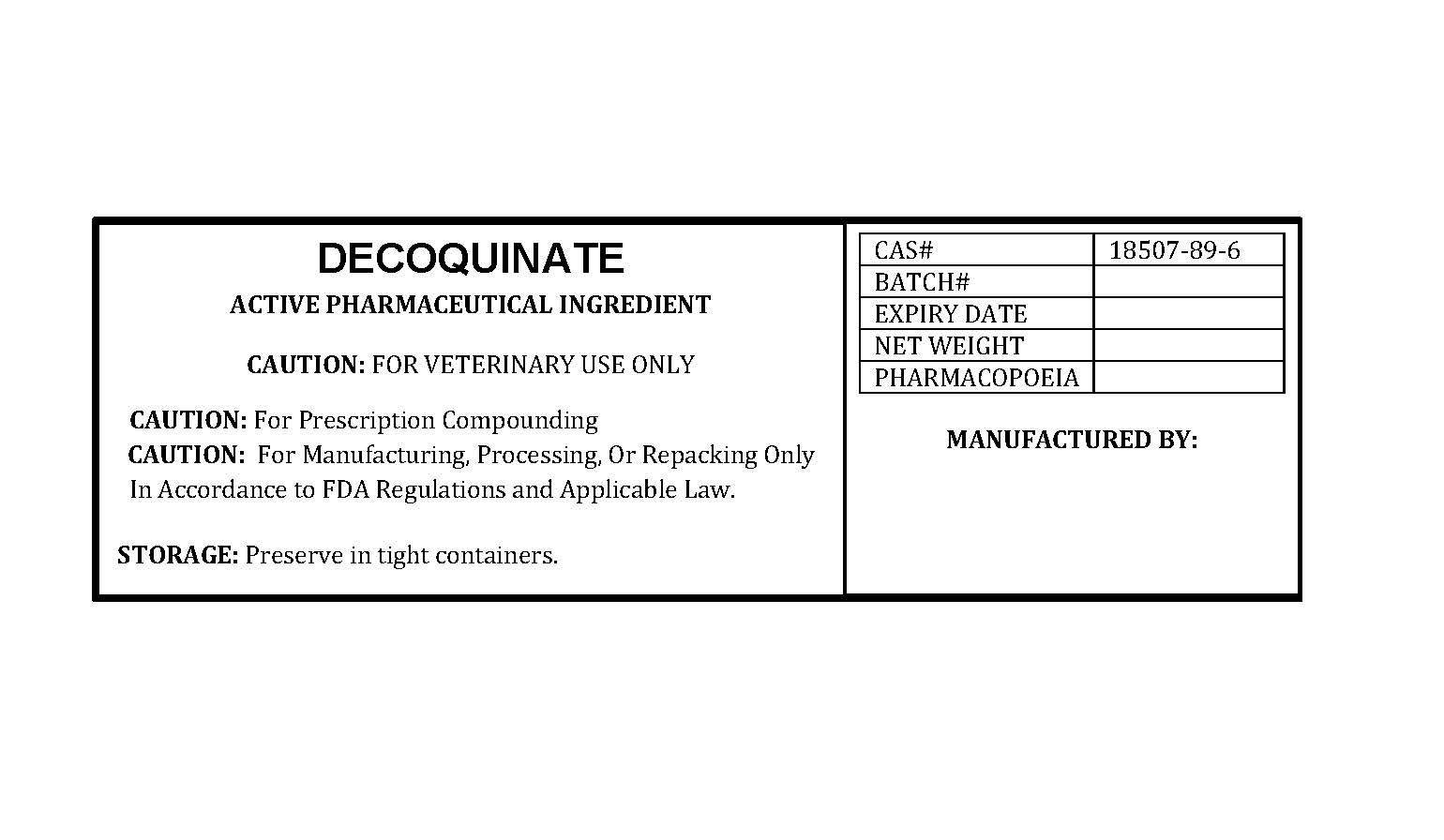
DECOQUINATE
DECOQUINATE
Approved
Approval ID
856a7a55-64d7-4cc5-ac0b-54020cbdbc20
Product Type
BULK INGREDIENT - ANIMAL DRUG
Effective Date
Aug 20, 2025
Manufacturers
FDA
MAEDA INC
DUNS: 118984873
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
DECOQUINATE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
NDC Product Code86184-063
Product Classification
G
Generic Name
DECOQUINATE
Product Specifications
Route of AdministrationNOT APPLICABLE
Effective DateAugust 20, 2025
FDA Product Classification
INGREDIENTS (1)
DECOQUINATEActive
Quantity: 1 g in 1 g
Code: 534I52PVWH
Classification: ACTIB
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
LOINC: 51945-4Updated: 8/20/2025
decoquinatelabelfile.jpg