Azelaic Acid Gel
These highlights do not include all the information needed to use AZELAIC ACID GEL safely and effectively. See full prescribing information for AZELAIC ACID GEL. AZELAIC ACID gel, for topical use Initial U.S. Approval: 1995
66178511-5c1a-4ecd-bc98-2ceb54e710ac
HUMAN PRESCRIPTION DRUG LABEL
Nov 11, 2021
Sandoz Inc.
DUNS: 005387188
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
azelaic acid
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC 0781-7172-50
Azelaic Acid Gel
15%
Rx Only
50 grams
SANDOZ
A Novartis Division

INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
Azelaic acid gel, 15% is indicated for topical treatment of the inflammatory papules and pustules of mild to moderate rosacea.
Limitations of Use
Although some reduction of erythema which was present in patients with papules and pustules of rosacea occurred in clinical studies, efficacy for treatment of erythema in rosacea in the absence of papules and pustules has not been evaluated.
Azelaic acid gel, 15% is indicated for topical treatment of the inflammatory papules and pustules of mild to moderate rosacea (1).
Limitations of Use
Efficacy for treatment of erythema in rosacea in the absence of papules and pustules has not been evaluated. (1)
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
None.
None. (4)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In two vehicle-controlled and one active-controlled U.S. clinical trials, treatment safety was monitored in 788 subjects who used twice-daily azelaic acid gel for 12 weeks (N=333) or 15 weeks (N=124), or the gel vehicle (N=331) for 12 weeks. In all three trials, the most common treatment-related adverse events were: burning/stinging/tingling (29%), pruritus (11%), scaling/dry skin/xerosis (8%) and erythema/irritation (4%). In the active-controlled trial, overall adverse reactions (including burning, stinging/tingling, dryness/tightness/scaling, itching, and erythema/irritation/redness) were 19.4% (24/124) for azelaic acid gel compared to 7.1% (9/127) for the active comparator gel at 15 weeks.
Table 1: Adverse Events Occurring in ≥1% of Subjects in the Rosacea Trials by Treatment Group and Maximum Intensity*|
Azelaic Acid Gel, 15% |
Vehicle | |||||
|---|---|---|---|---|---|---|
|
Mild |
Moderate |
Severe |
Mild |
Moderate |
Severe | |
| ||||||
|
Burning/stinging/tingling |
71 (16%) |
42 (9%) |
17 (4%) |
8 (2%) |
6 (2%) |
2 (1%) |
|
Pruritus |
29 (6%) |
18 (4%) |
5 (1%) |
9 (3%) |
6 (2%) |
0 (0%) |
|
Scaling/dry skin/xerosis |
21 (5%) |
10 (2%) |
5 (1%) |
31 (9%) |
14 (4%) |
1 (<1%) |
|
Erythema/irritation |
6 (1%) |
7 (2%) |
2 (<1%) |
8 (2%) |
4 (1%) |
2 (1%) |
|
Contact dermatitis |
2 (<1%) |
3 (1%) |
0 (0%) |
1 (<1%) |
0 (0%) |
0 (0%) |
|
Edema |
3 (1%) |
2 (<1%) |
0 (0%) |
3 (1%) |
0 (0%) |
0 (0%) |
|
Acne |
3 (1%) |
1 (<1%) |
0 (0%) |
1 (<1%) |
0 (0%) |
0 (0%) |
In patients using azelaic acid formulations, the following adverse events have been reported: worsening of asthma, vitiligo, depigmentation, small depigmented spots, hypertrichosis, reddening (signs of keratosis pilaris) and exacerbation of recurrent herpes labialis.
Local Tolerability Studies
Azelaic acid gel and its vehicle caused irritant reactions at the application site in human dermal safety studies. Azelaic acid gel caused significantly more irritation than its vehicle in a cumulative irritation study. Some improvement in irritation was demonstrated over the course of the clinical trials, but this improvement might be attributed to subject dropouts. No phototoxicity or photoallergenicity were reported in human dermal safety studies.
6.2 Postmarketing Experience
The following adverse reactions have been identified post approval of azelaic acid gel. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate the frequency or establish a causal relationship to drug exposure:
Eyes: iridocyclitis upon accidental exposure of the eyes to azelaic acid gel
Hypersensitivity: angioedema, eye swelling, facial swelling, urticaria.
Respiratory: worsening of asthma, dyspnea, wheezing.
Skin reactions: application site rash.
The most common adverse reactions are burning/stinging/tingling (29%), pruritus (11%), scaling/dry skin/xerosis (8%) and erythema/irritation (4%). (6)
To report SUSPECTED ADVERSE REACTIONS, contact LEO Pharma Inc. at 1-877-494-4536 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
Azelaic acid gel, 15% is a white to yellowish white opaque gel. Each gram of azelaic acid gel contains 0.15 gm of azelaic acid (15% w/w).
Gel, 15% (3)
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
- Cleanse affected area(s) using only very mild soaps or soapless cleansing lotion and pat dry with a soft towel before application of azelaic acid gel.
- Apply and gently massage a thin layer of azelaic acid gel into the affected areas on the face twice daily (morning and evening).
- Wash hands immediately following application of azelaic acid gel.
- Cosmetics may be applied after the application of azelaic acid gel has dried.
- Reassess the diagnosis if no improvement is observed upon completing 12 weeks of therapy.
- Avoid the use of occlusive dressings or wrappings.
- Instruct patients to avoid use of alcoholic cleansers, tinctures and astringents, abrasives and peeling agents.
- For topical use.
- Not for oral, ophthalmic or intravaginal use.
- Apply a thin layer twice daily to affected area(s). (2)
- Use only very mild soaps or soapless cleansing lotion and pat dry with a soft towel before applying azelaic acid gel. (2)
- Wash hands immediately following application. (2)
- Cosmetics may be applied after the application of azelaic acid gel has dried. (2)
- Avoid use of alcoholic cleansers, tinctures and astringents, abrasives and peeling agents. (2)
- For topical use. (2)
- Not for oral, ophthalmic or intravaginal use. (2)
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Azelaic acid is minimally absorbed systemically following topical route of administration, and maternal use is not expected to result in fetal exposure to the drug [see Clinical Pharmacology (12.3)].
In animal reproduction studies, embryofetal toxicity was noted when azelaic acid was administered orally during the period of organogenesis at doses 162, 19, and 65 times the maximum recommended human dose (MRHD) in rats, rabbits, and monkeys, respectively. Maternal toxicity was noted at these doses but no malformations were observed in these embryofetal developmental studies (see Data).
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
Dermal embryofetal developmental toxicology studies have not been performed with azelaic acid, 15% gel. Oral embryofetal developmental studies were conducted with azelaic acid in rats, rabbits, and cynomolgus monkeys. Azelaic acid was administered during the period of organogenesis in all three animal species. Embryotoxicity was observed in rats, rabbits, and monkeys at oral doses of azelaic acid that generated some maternal toxicity. Embryotoxicity was observed in rats given 2500 mg/kg/day [162 times the MRHD based on body surface area (BSA) comparison], rabbits given 150 or 500 mg/kg/day (19 or 65 times the MRHD based on BSA comparison) and cynomolgus monkeys given 500 mg/kg/day (65 times the MRHD based on BSA comparison) azelaic acid. No malformations were observed in the oral embryofetal developmental studies conducted in rats, rabbits and cynomolgus monkeys.
An oral peri- and post-natal developmental study was conducted in rats. Azelaic acid was administered from gestational day 15 through day 21 postpartum up to a dose level of 2500 mg/kg/day. Embryotoxicity was observed in rats at an oral dose of 2500 mg/kg/day (162 times the MRHD based on BSA comparison) that generated some maternal toxicity. In addition, slight disturbances in the post-natal development of fetuses was noted in rats at oral doses that generated some maternal toxicity (500 and 2500 mg/kg/day; 32 and 162 times the MRHD based on BSA comparison). No effects on sexual maturation of the fetuses were noted in this study.
8.2 Lactation
Risk Summary
Azelaic acid is naturally present in human milk. When used as prescribed, azelaic acid is unlikely to be absorbed through the skin in clinically relevant amounts to cause a change in azelaic acid concentration in milk or milk production; therefore, breastfeeding is not expected to result in exposure of the infant to azelaic acid gel. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for azelaic acid gel and any potential adverse effects on the breastfed child from azelaic acid gel or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of azelaic acid gel have not been established in pediatric patients.
8.5 Geriatric Use
Clinical studies of azelaic acid gel did not include sufficient numbers of subjects aged 65 and over to determine if they respond differently than younger subjects.
DESCRIPTION SECTION
11 DESCRIPTION
Azelaic acid gel, 15%, is an aqueous gel which contains azelaic acid, a naturally-occurring saturated dicarboxylic acid. It is for topical use. Chemically, azelaic acid is 1,7-heptanedicarboxylic acid. The molecular formula for azelaic acid is C9 H16 O4. It has the following structure:
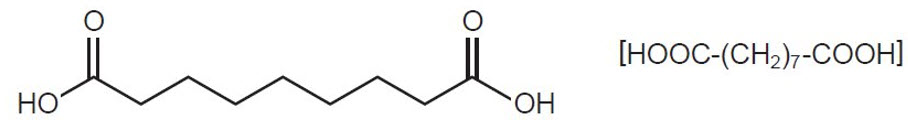
Azelaic acid has a molecular weight of 188.22. It is a white, odorless crystalline solid. It is poorly soluble in water at 20°C (0.24%) but freely soluble in boiling water and in ethanol.
Azelaic acid gel, 15% is a white to yellowish white opaque gel for topical use; each gram contains 0.15 gm azelaic acid (15% w/w) in an aqueous gel base containing benzoic acid (as a preservative), disodium EDTA, lecithin, medium- chain triglycerides, polyacrylic acid, polysorbate 80, propylene glycol, purified water, and sodium hydroxide to adjust pH.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism(s) by which azelaic acid interferes with the pathogenic events in rosacea are unknown.
12.2 Pharmacodynamics
The pharmacodynamics of azelaic acid in association with the treatment of rosacea are unknown.
12.3 Pharmacokinetics
The percutaneous absorption of azelaic acid after topical application of azelaic acid gel could not be reliably determined. Mean plasma azelaic acid concentrations in rosacea subjects treated with azelaic acid gel twice daily for at least 8 weeks are in the range of 42 to 63.1 ng/mL. These values are within the maximum concentration range of 24.0 to 90.5 ng/mL observed in rosacea subjects treated with vehicle only. This indicates that azelaic acid gel does not increase plasma azelaic acid concentration beyond the range derived from nutrition and endogenous metabolism.
In vitro and human data suggest negligible cutaneous metabolism of 3H-azelaic acid after topical application of 20% azelaic acid cream. Azelaic acid is mainly excreted unchanged in the urine, but undergoes some ß-oxidation to shorter chain dicarboxylic acids.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 2-year dermal mouse carcinogenicity study, azelaic acid pre-foam emulsion was administered twice daily to CD-1 mice at topical doses of 5%, 15%, and 30% (500, 1500, and 3000 mg/kg/day azelaic acid). No drug-related tumors were noted at concentrations up to 30% azelaic acid (396 times the MRHD based on AUC comparison).
Azelaic acid was not mutagenic or clastogenic in a battery of in vitro [Ames assay, HGPRT in V79 cells (Chinese hamster lung cells), and chromosomal aberration assay in human lymphocytes] and in vivo (dominant lethal assay in mice and mouse micronucleus assay) genotoxicity tests.
Oral administration of azelaic acid at dose levels up to 2500 mg/kg/day (162 times the MRHD based on BSA comparison) did not affect fertility or reproductive performance in male or female rats.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
Azelaic acid gel was evaluated for the treatment of mild to moderate papulopustular rosacea in two multicenter, randomized, double-blind, vehicle- controlled, 12-week clinical trials having identical protocols and involving a total of 664 (active: 333; vehicle: 331) subjects aged 21 to 86 years (mean age = 49). Overall, 92.5% of subjects were Caucasian and 73% of subjects were female. Enrolled subjects had mild to moderate rosacea with a mean lesion count of 18 (range 8 to 60) inflammatory papules and pustules. The following subjects were excluded: a) those without papules and pustules; b) those with nodules, rhinophyma, or ocular involvement and c) those with a history of hypersensitivity to propylene glycol or to any other ingredients of the study drug. Azelaic acid gel or its vehicle were to be applied twice daily for 12 weeks; no other topical or systemic medication affecting the course of rosacea and/or evaluability was to be used during the studies. Subjects were instructed to avoid spicy foods, thermally hot food/drink and alcoholic beverages during the study. Subjects were also instructed to use only very mild soaps or soapless cleansing lotion for facial cleansing.
The primary efficacy endpoints included both 1) change from baseline in inflammatory lesion counts as well as 2) success defined as a score of "clear" or "minimal" with at least a 2-step reduction from baseline on the Investigator's Global Assessment (IGA), defined as follows below:
CLEAR:
No papules and/or pustules; no or residual erythema; no or mild to moderate telangiectasia
MINIMAL:
Rare papules and/or pustules; residual to mild erythema; mild to moderate telangiectasia
MILD:
Few papules and/or pustules; mild erythema; mild to moderate telangiectasia
MILD TO MODERATE:
Distinct number of papules and/or pustules; mild to moderate erythema; mild to moderate telangiectasia
MODERATE:
Pronounced number of papules and/or pustules; moderate erythema; mild to moderate telangiectasia
MODERATE TO SEVERE:
Many papules and/or pustules, occasionally with large inflamed lesions; moderate erythema; moderate degree of telangiectasia
SEVERE:
Numerous papules and/or pustules, occasionally with confluent areas of inflamed lesions; moderate or severe erythema; moderate or severe telangiectasia
Primary efficacy assessment was based on the "intent-to-treat" (ITT) population with the "last observation carried forward" (LOCF).
Both trials demonstrated a statistically significant difference in favor of azelaic acid gel over its vehicle in both reducing the number of inflammatory papules and pustules associated with rosacea (Table 2) as well as demonstrating success on the IGA in the ITT-LOCF population at the end of treatment.
Table 2: Inflammatory Papules and Pustules (ITT population)*|
Study One |
Study One |
Study Two |
Study Two | |
|---|---|---|---|---|
| ||||
|
Mean Lesion Count Baseline |
17.5 |
17.6 |
17.9 |
18.5 |
|
End of Treatment* |
6.8 |
10.5 |
9.0 |
12.1 |
|
Mean Percent Reduction End of Treatment* |
57.9% |
39.9% |
50.0% |
38.2% |
Although some reduction of erythema which was present in subjects with papules and pustules of rosacea occurred in clinical trials, efficacy for treatment of erythema in rosacea in the absence of papules and pustules has not been evaluated.
Azelaic acid gel was superior to the vehicle with regard to success based on the IGA of rosacea on a 7-point static score at the end of treatment (ITT population;Table 3).
Table 3: Investigator's Global Assessment at the End of Treatment*|
Study One |
Study One |
Study Two |
Study Two | |
|---|---|---|---|---|
| ||||
|
Clear, Minimal or Mild at End of Treatment |
61% |
40% |
61% |
48% |
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
Azelaic acid gel, 15% is a white to yellowish white opaque gel supplied in a 50 g tube (NDC 0781-7172-50).
Storage and Handling
Store at 25°C (77°F); excursions permitted between 15-30°C (59-86°F) [see USP Controlled Room Temperature].
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Inform patients using azelaic acid gel of the following:
Administration Instructions
- For topical use only.
- Before applying azelaic acid gel, cleanse affected area(s) with a very mild soap or a soapless cleansing lotion and pat dry with a soft towel.
- Wash hands immediately following application of azelaic acid gel.
- Cosmetics may be applied after the application of azelaic acid gel has dried.
- Avoid the use of occlusive dressings or wrappings.
- Avoid use of alcoholic cleansers, tinctures and astringents, abrasives and peeling agents [see Dosage and Administration (2)].
Hypersensitivity
- If allergic reactions occur, discontinue use and consult their healthcare provider [see Warnings and Precautions (5.1)].
Skin Irritation
- Skin irritation (e.g., pruritus, burning, or stinging) may occur during use of azelaic acid gel, usually during the first few weeks of treatment. If irritation is excessive or persists, or allergic reactions occur, discontinue use and consult your physician [see Warnings and Precautions (5.2)].
Hypopigmentation
- Advise patients to report abnormal changes in skin color to their healthcare provider [see Warnings and Precautions (5.2)].
Eye and Mucous Membranes Irritation
- Avoid contact with the eyes, mouth and other mucous membranes. If azelaic acid gel comes in contact with the eyes, wash the eyes with large amounts of water and consult their healthcare provider if eye irritation persists [see Warnings and Precautions (5.3)].
Exacerbation of Asthma
- Advise patients to report any worsening of asthma to their healthcare provider [see Warnings and Precautions (5.4)].
SPL UNCLASSIFIED SECTION
© 2021, LEO Pharma Inc. All rights reserved.
Manufactured for
LEO Pharma A/S
Industriparken 55
Ballerup, 2750, Denmark
Distributed by
Sandoz Inc.
Princeton, NJ 08540
