Modafinil
{'content': {'@styleCode': 'bold', 'content': [{'@styleCode': 'bold', 'br': None, '#text': 'These highlights do not include all the information needed to use MODAFINIL TABLETS safely and effectively. See full prescribing information for MODAFINIL TABLETS.'}, {'@styleCode': 'bold', 'br': None, '#text': 'MODAFINIL tablets, for oral use, C-IV Initial U.S. Approval: 1998'}], 'br': [None, None, None]}}
944daf47-49a6-93ca-e348-4a0f1b6d937e
HUMAN PRESCRIPTION DRUG LABEL
Sep 19, 2025
Apotex Corp.
DUNS: 845263701
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
modafinil
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
modafinil
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
Drug Labeling Information
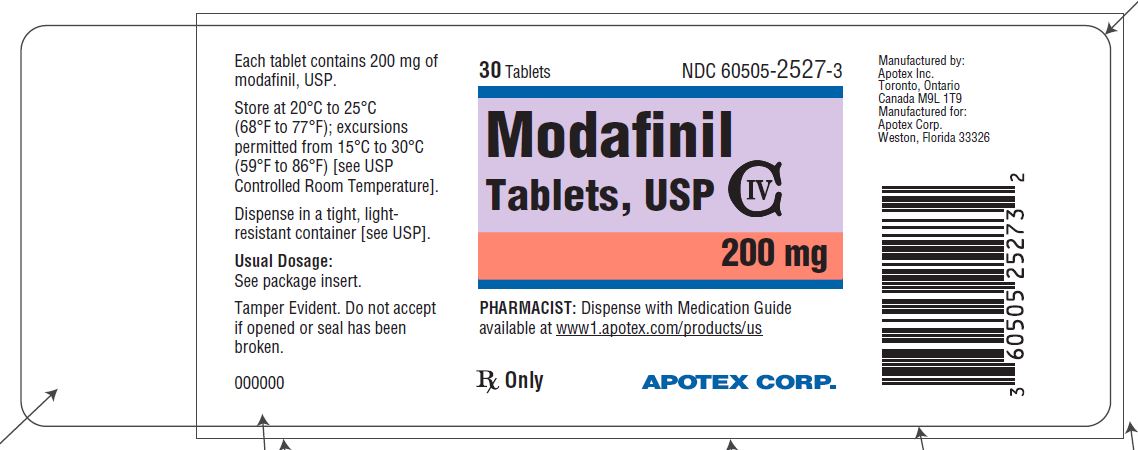
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Representative sample of labeling (see HOW SUPPLIED section for complete listing):
PRINCIPAL DISPLAY PANEL - 200 mg
APOTEX CORP. NDC 60505-2527-3
Modafinil Tablets
200 mg
Rx
30 count
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C
There are no adequate and well-controlled studies of modafinil in pregnant
women. Intrauterine growth restriction and spontaneous abortion have been
reported in association with modafinil (a mixture of R- and S-modafinil) and
armodafinil (the R-enantiomer of modafinil). Although the pharmacology of
modafinil is not identical to that of the sympathomimetic amines, it does
share some pharmacologic properties with this class. Certain of these drugs
have been associated with intrauterine growth restriction and spontaneous
abortions. Whether the cases reported with modafinil are drug-related is
unknown. In studies of modafinil and armodafinil conducted in rats (modafinil,
armodafinil) and rabbits (modafinil), developmental toxicity was observed at
clinically relevant plasma exposures. Modafinil tablets should be used during
pregnancy only if the potential benefit justifies the potential risk to the
fetus.
Modafinil (50, 100, or 200 mg/kg/day) administered orally to pregnant rats throughout organogenesis caused, in the absence of maternal toxicity, an increase in resorptions and an increased incidence of visceral and skeletal variations in the offspring at the highest dose tested. The higher no-effect dose for embryofetal developmental toxicity in rats (100 mg/kg/day) was associated with a plasma modafinil AUC less than that in humans at the recommended human dose (RHD) of modafinil tablets (200 mg/day). However, in a subsequent study of up to 480 mg/kg/day of modafinil, no adverse effects on embryofetal development were observed. Oral administration of armodafinil (60, 200, or 600 mg/kg/day) to pregnant rats throughout organogenesis resulted in increased incidences of fetal visceral and skeletal variations and decreased fetal body weight at the highest dose tested. The highest no-effect dose for embryofetal developmental toxicity in rats (200 mg/kg/day) was associated with a plasma armodafinil AUC less than that in humans at the RHD of modafinil tablets.
Modafinil administered orally to pregnant rabbits throughout organogenesis at doses of up to 100 mg/kg/day had no effect on embryofetal development; however, the doses used were too low to adequately assess the effects of modafinil on embryofetal development. In a subsequent developmental toxicity study evaluating doses of 45, 90, and 180 mg/kg/day in pregnant rabbits, the incidences of fetal structural alterations and embryofetal death were increased at the highest dose. The highest no-effect dose for developmental toxicity (100 mg/kg/day) was associated with a plasma modafinil AUC similar to that in humans at the RHD of modafinil tablets.
Modafinil administration to rats throughout gestation and lactation at oral doses of up to 200 mg/kg/day resulted in decreased viability in the offspring at doses greater than 20 mg/kg/day, a dose resulting in a plasma modafinil AUC less than that in humans at the RHD of modafinil tablets. No effects on postnatal developmental and neurobehavioral parameters were observed in surviving offspring.
8.3 Nursing Mothers
It is not known whether modafinil or its metabolites are excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when modafinil tablets are administered to a nursing woman.
8.4 Pediatric Use
Safety and effectiveness in pediatric patients have not been established. Modafinil tablets are not approved in this population for any indication.
Serious skin rashes, including erythema multiforme major (EMM) and Stevens- Johnson Syndrome (SJS) have been associated with modafinil use in pediatric patients [see Warnings and Precautions (5.1)].
In a controlled 6-week study, 165 pediatric patients (aged 5 to 17 years) with narcolepsy were treated with modafinil (n=123), or placebo (n=42). There were no statistically significant differences favoring modafinil over placebo in prolonging sleep latency as measured by MSLT, or in perceptions of sleepiness as determined by the clinical global impression-clinician scale (CGI-C).
In the controlled and open-label clinical studies, treatment emergent adverse reactions of the psychiatric and nervous system included Tourette’s syndrome, insomnia, hostility, increased cataplexy, increased hypnagogic hallucinations, and suicidal ideation. Transient leukopenia, which resolved without medical intervention, was also observed. In the controlled clinical study, 3 of 38 girls, ages 12 or older, treated with modafinil experienced dysmenorrhea compared to 0 of 10 girls who received placebo.
There were three 7 to 9 week, double-blind, placebo-controlled, parallel group studies in children and adolescents (aged 6 to 17 years) with Attention- Deficit Hyperactivity Disorder (ADHD). Two of the studies were flexible-dose studies (up to 425 mg/day), and the third was a fixed-dose study (340 mg/day for patients <30 kg and 425 mg/day for patients ≥30 kg). Although these studies showed statistically significant differences favoring modafinil over placebo in reducing ADHD symptoms as measured by the ADHD-RS (school version), there were 3 cases of serious rash including one case of possible SJS among 933 patients exposed to modafinil in this program. Modafinil is not approved for use in treating ADHD.
8.5 Geriatric Use
In clinical trials, experience in a limited number of modafinil-treated patients who were greater than 65 years of age showed an incidence of adverse reactions similar to other age groups. In elderly patients, elimination of modafinil and its metabolites may be reduced as a consequence of aging. Therefore, consideration should be given to the use of lower doses and close monitoring in this population [see Dosage and Administration (2.4) and Clinical Pharmacology (12.3)].
8.6 Hepatic Impairment
In patients with severe hepatic impairment, the dose of modafinil tablets should be reduced to one-half of that recommended for patients with normal hepatic function [see Dosage and Administration (2.3) and Clinical Pharmacology (12.3)].
Pregnancy: Based on animal data, may cause fetal harm. (8.1)
OVERDOSAGE SECTION
10 OVERDOSAGE
In clinical trials, a total of 151 protocol-specified doses ranging from 1000 to 1600 mg/day (5 to 8 times the recommended daily dose of modafinil tablets) have been administered to 32 subjects, including 13 subjects who received doses of 1000 or 1200 mg/day for 7 to 21 consecutive days. In addition, several intentional acute overdoses occurred; the two largest being 4500 mg and 4000 mg taken by two subjects participating in foreign depression studies. None of these study subjects experienced any unexpected or life-threatening effects. Adverse reactions that were reported at these doses included excitation or agitation, insomnia, and slight or moderate elevations in hemodynamic parameters. Other observed high-dose effects in clinical studies have included anxiety, irritability, aggressiveness, confusion, nervousness, tremor, palpitations, sleep disturbances, nausea, diarrhea, and decreased prothrombin time.
From postmarketing experience, there have been reports of fatal overdoses involving modafinil alone or in combination with other drugs. Symptoms most often accompanying modafinil tablets overdose, alone or in combination with other drugs have included insomnia; central nervous system symptoms such as restlessness, disorientation, confusion, agitation, anxiety, excitation, and hallucination; digestive changes such as nausea and diarrhea; and cardiovascular changes such as tachycardia, bradycardia, hypertension, and chest pain.
Cases of accidental ingestion/overdose have been reported in children as young as 11 months of age. The highest reported accidental ingestion on a mg/kg basis occurred in a three-year-old boy who ingested 800 to 1000 mg (50 to 63 mg/kg) of modafinil tablets. The child remained stable. The symptoms associated with overdose in children were similar to those observed in adults.
No specific antidote exists for the toxic effects of a modafinil tablets overdose. Such overdoses should be managed with primarily supportive care, including cardiovascular monitoring.
DESCRIPTION SECTION
11 DESCRIPTION
Modafinil, USP is a wakefulness-promoting agent for oral administration. Modafinil, USP is a racemic compound. The chemical name for modafinil is 2-[(diphenylmethyl)sulfinyl]acetamide. The molecular formula is C15H15NO2S and the molecular weight is 273.35.
The chemical structure is:
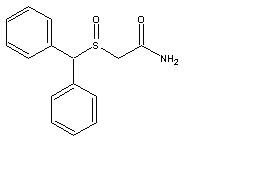
Modafinil, USP is a white to off-white, crystalline powder that is practically insoluble in water and cyclohexane. It is sparingly to slightly soluble in methanol and acetone.
Modafinil tablets, USP contain 100 mg or 200 mg of modafinil, USP and the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, magnesium stearate and methylcellulose.
Modafinil Tablets meet USP Dissolution Test 3.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Allergic Reactions
Advise patients to stop taking modafinil tablets and to notify their physician
right away if they develop a rash, hives, mouth sores, blisters, peeling skin,
trouble swallowing or breathing, or a related allergic phenomenon.
Driving and Dangerous Activities
Advise patients not to alter their previous behavior with regard to
potentially dangerous activities (e.g., driving, operating machinery) or other
activities requiring appropriate levels of wakefulness, until and unless
treatment with modafinil tablets have been shown to produce levels of
wakefulness that permit such activities. Advise patients that modafinil tablet
is not a replacement for sleep.
Continuing Previously Prescribed Treatments
Inform patients that it may be critical that they continue to take their
previously prescribed treatments (e.g., patients with OSA receiving CPAP
should continue to do so).
Discontinuing Drug Due to Adverse Reactions
Advise patients to stop taking modafinil tablets and contact their physician
right away if they experience chest pain, rash, depression, anxiety, or signs
of psychosis or mania.
Pregnancy
Advise patients to notify their physician if they become pregnant or intend to
become pregnant during therapy. Caution patients regarding the potential
increased risk of pregnancy when using steroidal contraceptives (including
depot or implantable contraceptives) with modafinil tablets and for one month
after discontinuation of therapy.
Nursing
Advise patients to notify their physician if they are breastfeeding an infant.
Concomitant Medication
Advise patients to inform their physician if they are taking, or plan to take,
any prescription or over-the-counter drugs, because of the potential for
interactions between modafinil tablets and other drugs.
Alcohol
Advise patients that the use of modafinil tablets in combination with alcohol
has not been studied. Advise patients that it is prudent to avoid alcohol
while taking modafinil tablets.
Dispense with Medication Guide available at www1.apotex.com/products/us
APOTEX INC.
MODAFINIL TABLETS, USP
100 mg and 200 mg
|
Manufactured by |
Manufactured for |
|
Apotex Inc. |
Apotex Corp. |
|
Toronto, Ontario |
Weston, Florida |
|
Canada M9L 1T9 |
33326 |
Revised: January 2022
Rev. 10
SPL MEDGUIDE SECTION
Medication Guide
Modafinil Tablets, USP

100 mg and 200 mg
(moe daf' i nil)****
Rx Only
Medication Guide available at www1.apotex.com/products/us
Read this Medication Guide before you start taking modafinil tablets and each time you get a refill. There may be new information. This information does not take the place of talking with your doctor about your medical condition or treatment.
What is the most important information I should know about modafinil tablets?
Modafinil tablets may cause serious side effects including a serious rash or a serious allergic reaction that may affect parts of your body such as your liver or blood cells. Any of these may need to be treated in a hospital and may be life-threatening.
Stop taking modafinil tablets and call your doctor right away or get emergency help if you have any of these symptoms:
- skin rash, hives, sores in your mouth, or your skin blisters and peels
- swelling of your face, eyes, lips, tongue, or throat
- trouble swallowing or breathing
- fever, shortness of breath, swelling of the legs, yellowing of the skin or whites of the eyes, or dark urine.
If you have a severe rash with modafinil tablets, stopping the medicine may not keep the rash from becoming life-threatening or causing you to be permanently disabled or disfigured.
Modafinil tablets are not approved for use in children for any medical
condition.
It is not known if modafinil tablets are safe or effective in children under
17 years of age.
What are modafinil tablets?
Modafinil tablets are a prescription medicine used to improve wakefulness in adults who are very sleepy due to one of the following diagnosed sleep disorders:
- narcolepsy
- obstructive sleep apnea (OSA). Modafinil tablets are used to treat excessive sleepiness, but not the obstruction or medical condition that is causing OSA. You should talk with your doctor about treatments for OSA before you start taking modafinil tablets and during treatment with modafinil tablets. Modafinil tablets do not take the place of treatments that your doctor has prescribed for OSA. It is important that you continue to use these treatments as prescribed by your doctor.
- shift work disorder (SWD)
Modafinil tablets will not cure these sleep disorders. Modafinil tablets may help the sleepiness caused by these conditions, but it may not stop all your sleepiness. Modafinil tablets do not take the place of getting enough sleep. Follow your doctor’s advice about good sleep habits and using other treatments.
|
Modafinil is a federally controlled substance (C-IV) because it can be abused or lead to dependence. Keep modafinil tablets in a safe place to prevent misuse and abuse. Selling or giving away modafinil tablets may harm others, and is against the law. Tell your doctor if you have ever abused or been dependent on alcohol, prescription medicines or street drugs. |
Who should not take modafinil tablets?
Do not take modafinil tablets if you:
- are allergic or developed a rash to modafinil or armodafinil (NUVIGIL® ) or any of the ingredients in modafinil tablets. See the end of this Medication Guide for a complete list of ingredients in modafinil tablets.
What should I tell my doctor before taking modafinil tablets?
Tell your doctor about all of your medical conditions including, if you:
- have a history of mental health problems, including psychosis
- have heart problems or had a heart attack
- have high blood pressure. Your blood pressure may need to be checked more often while taking modafinil tablets.
- have liver or kidney problems
- have a history of drug or alcohol abuse or addiction
- are pregnant or planning to become pregnant. It is not known if modafinil will harm your unborn baby.
- are breastfeeding. It is not known if modafinil passes into your breast milk. Talk to your doctor about the best way to feed your baby if you take modafinil tablets.
**Tell your doctor about all the medicines you take,**including prescription and over-the-counter medicines, vitamins, and herbal supplements. Modafinil tablets and many other medicines can interact with each other, sometimes causing side effects. Modafinil tablets may affect the way other medicines work, and other medicines may affect how modafinil tablets works. Your dose of modafinil tablets or certain other medicines may need to be changed.
Especially, tell your doctor if you use or take:
- a hormonal birth control method, such as birth control pills, shots, implants, patches, vaginal rings, and intrauterine devices (IUDs). Hormonal birth control methods may not work while you take modafinil tablets. Women who use one of these methods of birth control may have a higher chance for getting pregnant while taking modafinil tablets, and for one month after stopping modafinil tablets. Talk to your doctor about birth control choices that are right for you while taking modafinil tablets.
Know the medicines you take. Keep a list of them and show it to your doctor and pharmacist when you get a new medicine. Your doctor or pharmacist will tell you if it is safe to take modafinil tablets and other medicines together. Do not start any new medicines with modafinil tablets unless your doctor has told you it is okay.
How should I take modafinil tablets?
- Take modafinil tablets exactly as prescribed by your doctor. Your doctor will prescribe the dose of modafinil tablets that is right for you. Do not change your dose of modafinil tablets without talking to your doctor.
- Your doctor will tell you the right time of day to take modafinil tablets.
- People with narcolepsy or OSA usually take modafinil tablets one time each day in the morning.
- People with SWD usually take modafinil tablets about 1 hour before their work shift.
- Do not change the time of day you take modafinil tablets unless you have talked to your doctor. If you take modafinil tablets too close to your bedtime, you may find it harder to go to sleep.
- You can take modafinil tablets with or without food.
- If you take more than your prescribed dose or if you take an overdose of modafinil tablets, call your doctor or go to the nearest hospital emergency room right away.
Symptoms of an overdose of modafinil tablets may include:
- trouble sleeping
- restlessness
- confusion
- feeling disoriented
- feeling excited
- hearing, seeing, feeling, or sensing things that are not really there (hallucinations)
- nausea and diarrhea
- a fast or slow heartbeat
- chest pain
- increased blood pressure
What should I avoid while taking modafinil tablets?
- Do not drive a car or do other dangerous activities until you know how modafinil tablets affect you. People with sleep disorders should always be careful about doing things that could be dangerous. Do not change your daily habits until your doctor tells you it is okay.
- You should avoid drinking alcohol. It is not known how drinking alcohol will affect you when taking modafinil tablets.
What are possible side effects of modafinil tablets?
**Modafinil tablets may cause serious side effects.**Stop taking modafinil tablets and call your doctor right away or get emergency help if you get any of the following:
a serious rash or serious allergic reaction.(See*"What is the most important information I should know about modafinil tablets?"**) *mental (psychiatric) symptoms, including: * depression * feeling anxious * hearing, seeing, feeling, or sensing things that are not really there (hallucinations) * an extreme increase in activity and talking (mania) * thoughts of suicide * aggressive behavior * other mental problems ***symptoms of a heart problem,**including chest pain, abnormal heartbeat, and trouble breathing.
Common side effects that can happen in anyone who takes modafinil tablets include:
- headache
- back pain
- nausea
- feeling nervous
- stuffy nose
- diarrhea
- feeling anxious
- trouble sleeping
- dizziness
- upset stomach
Modafinil tablets are not approved for use in children for any medical condition including Attention Deficit Hyperactivity Disorder (ADHD). In studies of modafinil tablets in children with narcolepsy, side effects included:
- Tourette’s syndrome
- hostile behavior
- increase in sudden loss of muscle tone and severe muscle weakness
- increase in seeing and hearing things when falling asleep
- increase in suicidal thoughts
- low white blood count
- painful menstrual periods
Tell your doctor if you get any side effect that bothers you or that does not go away while taking modafinil tablets.
These are not all the side effects of modafinil tablets. For more information, ask your doctor or pharmacist.
Some effects of modafinil tablets on the brain are the same as other medicines called "stimulants". These effects may lead to abuse or dependence on modafinil tablets.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store modafinil tablets?
- Store modafinil tablets at room temperature between 20°C to 25°C (68°F to 77°F); excursions permitted from 15°C to 30°C (59°F to 86°F). *Keep modafinil tablets and all medicines out of the reach of children.
General information about the safe and effective use of modafinil tablets
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use modafinil tablets for a condition for which it was not prescribed.****Do not give modafinil tablets to other people, even if they have the same symptoms you have. It may harm them and it is against the law.
This Medication Guide summarizes the most important information about modafinil tablets. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about modafinil tablets that is written for health professionals. For more information, call Apotex Corp. at 1-800-706-5575.
What are the ingredients in modafinil tablets?
**Active Ingredient:**modafinil
**Inactive Ingredients:**colloidal silicon dioxide, croscarmellose sodium,
magnesium stearate and methylcellulose.
All registered trademarks in this document are the property of their respective owners.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
APOTEX INC.
MODAFINIL TABLETS, USP
100 mg and 200 mg
|
Manufactured by |
Manufactured for |
|
Apotex Inc. |
Apotex Corp. |
|
Toronto, Ontario |
Weston, Florida |
|
Canada M9L 1T9 |
33326 |
Revised: January 2022
Rev. 10
