Piroxicam
These highlights do not include all the information needed to use PIROXICAM CAPSULES safely and effectively. See full prescribing information for PIROXICAM CAPSULES. PIROXICAM capsules, for oral useInitial U.S. Approval: 1982
d9684cb2-76f3-4c1b-b9e5-1f8fe55f3695
HUMAN PRESCRIPTION DRUG LABEL
Mar 11, 2024
Bryant Ranch Prepack
DUNS: 171714327
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
piroxicam
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Piroxicam 10 mg cap#100
Boxed Warning section
WARNING: RISK OF SERIOUS CARDIOVASCULAR AND GASTROINTESTINAL EVENTS
See full prescribing information for complete boxed warning.
•
**Nonsteroidal anti-inflammatory drugs (NSAIDs) cause an increased risk of serious cardiovascular thrombotic events, including myocardial infarction and stroke, which can be fatal. This risk may occur early in treatment and may increase with duration of use (****5.1****)**
•
**Piroxicam capsules are contraindicated in the setting of coronary artery bypass graft (CABG) surgery (****4****,****5.1****)**
•
**NSAIDs cause an increased risk of serious gastrointestinal (GI) adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients and patients with a prior history of peptic ulcer disease and/or GI bleeding are at greater risk for serious GI events (****5.2****)**
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
Piroxicam capsules are indicated:
•
For relief of the signs and symptoms of osteoarthritis.
•
For relief of the signs and symptoms of rheumatoid arthritis.
Piroxicam capsules are a nonsteroidal anti-inflammatory drug indicated for
•Relief of the signs and symptoms of osteoarthritis (OA) (1)•Relief of the signs and symptoms of rheumatoid arthritis (RA) (1)
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
Piroxicam capsules are contraindicated in the following patients:
•
Known hypersensitivity (e.g., anaphylactic reactions and serious skin reactions) to piroxicam or any components of the drug product [see Warnings and Precautions (5.7, 5.9)]
•
History of asthma, urticaria, or other allergic-type reactions after taking aspirin or other NSAIDs. Severe, sometimes fatal, anaphylactic reactions to NSAIDs have been reported in such patients [see Warnings and Precautions (5.7, 5.8)]
•
In the setting of CABG surgery [see Warnings and Precautions (5.1)]
•
Known hypersensitivity to piroxicam or any components of the drug product (4)
•
History of asthma, urticaria, or other allergic-type reactions after taking aspirin or other NSAIDs (4)
•
In the setting of CABG surgery (4)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling:
•
Cardiovascular Thrombotic Events [see Warnings and Precautions (5.1)]
•
GI Bleeding, Ulceration and Perforation [see Warnings and Precautions (5.2)]
•
Hepatotoxicity [see Warnings and Precautions (5.3)]
•
Hypertension [see Warnings and Precautions (5.4)]
•
Heart Failure and Edema [see Warnings and Precautions (5.5)]
•
Renal Toxicity and Hyperkalemia [see Warnings and Precautions (5.6)]
•
Anaphylactic Reactions [see Warnings and Precautions (5.7)]
•
Serious Skin Reactions [see Warnings and Precautions (5.9)]
•
Hematologic Toxicity [see Warnings and Precautions (5.11)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In patients taking piroxicam capsules or other NSAIDs, the most frequently reported adverse experiences occurring in approximately 1% to 10% of patients are:
**Cardiovascular System:**Edema
**Digestive System:**Anorexia, abdominal pain, constipation, diarrhea, flatulence, nausea, vomiting
**Nervous System:**Dizziness, headache, vertigo
**Skin and Appendages:**Pruritus, rash
**Special Senses:**Tinnitus
Additional adverse experiences reported occasionally include:
**Cardiovascular System:**Palpitations
**Digestive System:**Stomatitis
**Nervous System:**Drowsiness
**Special Senses:**Blurred vision
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of piroxicam capsules. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
**Body As a Whole:**Fever, infection, sepsis, anaphylactic reactions, appetite changes, death, flu-like syndrome, pain (colic), serum sickness
**Cardiovascular System:**Congestive heart failure, hypertension, tachycardia, syncope, arrhythmia, exacerbation of angina, hypotension, myocardial infarction, vasculitis
**Digestive System:**Dyspepsia, elevated liver enzymes, gross bleeding/perforation, heartburn, ulcers (gastric/duodenal), dry mouth, esophagitis, gastritis, glossitis, hematemesis, hepatitis, jaundice, melena, rectal bleeding, eructation, liver failure, pancreatitis
**Hemic and Lymphatic System:**Anemia, increased bleeding time, ecchymosis, eosinophilia, epistaxis, leukopenia, purpura, petechial rash, thrombocytopenia, agranulocytosis, hemolytic anemia, aplastic anemia, lymphadenopathy, pancytopenia
Hypersensitivity: Positive ANA
**Metabolic and Nutritional:**Weight changes, Fluid retention, hyperglycemia, hypoglycemia
**Nervous System:**Anxiety, asthenia, confusion, depression, dream abnormalities, insomnia, malaise, nervousness, paresthesia, somnolence, tremors, akathisia, convulsions, coma, hallucinations, meningitis, mood alterations
**Respiratory System:**Asthma, dyspnea, respiratory depression, pneumonia
**Skin and Appendages:**Alopecia, bruising, desquamation, erythema, photosensitivity, sweat, angioedema, toxic epidermal necrosis, erythema multiforme, exfoliative dermatitis, onycholysis, Stevens Johnson Syndrome, urticaria, vesiculobullous reaction
**Special Senses:**Conjunctivitis, hearing impairment, swollen eyes
**Urogenital System:**Abnormal renal function, cystitis, dysuria, hematuria, hyperkalemia, interstitial nephritis, nephrotic syndrome, oliguria/polyuria, proteinuria, renal failure, glomerulonephritis
**Reproductive System and Breast Disorders:**Female fertility decreased
Most common adverse reactions (incidence > 2% from clinical trials) are: nausea, constipation, flatulence, abdominal pain, diarrhea, headache, dizziness, edema, rash. (6.1)
**To report SUSPECTED ADVERSE REACTIONS, contact Nostrum Laboratories, Inc. at quality@nostrumpharma.com or 1-877-770-1288 or FDA at 1-800-FDA-1088 or **www.fda.gov/medwatch.
RECENT MAJOR CHANGES SECTION
RECENT MAJOR CHANGES
Warnings and Precautions,
Gastrointestinal Bleeding, Ulceration, and Perforation (5.2) 05/2019
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
Carefully consider the potential benefits and risks of piroxicam capsules and other treatment options before deciding to use piroxicam capsules. Use the lowest effective dosage for the shortest duration consistent with individual patient treatment goals [see Warnings and Precautions (5)].
After observing the response to initial therapy with piroxicam capsules, the dose and frequency should be adjusted to suit an individual patient’s needs.
For the relief of rheumatoid arthritis and osteoarthritis, the dosage is 20 mg given orally once per day. If desired, the daily dose may be divided. Because of the long half-life of piroxicam capsules, steady-state blood levels are not reached for 7 to 12 days. Therefore, although the therapeutic effects of piroxicam capsules are evident early in treatment, there is a progressive increase in response over several weeks and the effect of therapy should not be assessed for two weeks.
•
Use the lowest effective dosage for shortest duration consistent with individual patient treatment goals (2)
•
OA and RA: 20 mg once daily (2)
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
Piroxicam Capsules, USP are available containing 10 mg or 20 mg of piroxicam, USP.
•
The 10 mg capsule is a hard gelatin capsule with a swedish orange opaque cap and ivory opaque body containing white to off-white powder. The capsule is imprinted with**NP**above**10**in black ink on the cap.
•
The 20 mg capsule is a hard gelatin capsule with a swedish orange opaque cap and swedish orange opaque body containing white to off-white powder. The capsule is imprinted with**NP**above**20**in black ink on the cap.
Piroxicam capsules: 10 mg and 20 mg (3)
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Use of NSAIDs, including piroxicam capsules, during the third trimester of pregnancy increases the risk of premature closure of the fetal ductus arteriosus. Avoid use of NSAIDs, including piroxicam capsules, in pregnant women starting at 30 weeks of gestation (third trimester).
There are no adequate and well-controlled studies of piroxicam capsules in pregnant women.
Data from observational studies regarding potential embryofetal risks of NSAID use in women in the first or second trimesters of pregnancy are inconclusive. In the general U.S. population, all clinically recognized pregnancies, regardless of drug exposure, have a background rate of 2% to 4% for major malformations, and 15% to 20% for pregnancy loss. In animal reproduction studies in rats and rabbits, there was no evidence of teratogenicity at exposures up to 5 and 10 times the maximum recommended human dose (MRHD), respectively. In rat studies with piroxicam, fetotoxicity (postimplantation loss) was observed at exposures 2 times the MRHD, and delayed parturition and an increased incidence of stillbirth were noted at doses equivalent to the MRHD of piroxicam. Based on animal data, prostaglandins have been shown to have an important role in endometrial vascular permeability, blastocyst implantation, and decidualization. In animal studies, administration of prostaglandin synthesis inhibitors such as piroxicam, resulted in increased pre- and post-implantation loss.
Clinical Considerations
Labor or Delivery
There are no studies on the effects of piroxicam capsules during labor or delivery. In animal studies, NSAIDs, including piroxicam inhibit prostaglandin synthesis, cause delayed parturition, and increase the incidence of stillbirth.
Data
Animal Data
Pregnant rats administered piroxicam at 2, 5, or 10 mg/kg/day during the period of organogenesis (Gestation Days 6 to 15) demonstrated increased post- implantation losses with 5 and 10 mg/kg/day of piroxicam (equivalent to 2 and 5 times the MRHD, of 20 mg respectively, based on a mg/m2 body surface area [BSA]). There were no drug-related developmental abnormalities noted in offspring. Gastrointestinal tract toxicity was increased in pregnant rats in the last trimester of pregnancy compared to non-pregnant rats or rats in earlier trimesters of pregnancy. Pregnant rabbits administered piroxicam at 2, 5, or 10 mg/kg/day during the period of organogenesis (Gestation Days 7 to 18) demonstrated no drug-related developmental abnormalities in offspring (up to 10 times the MRHD based on a mg/m2 BSA).
In a pre- and post-natal development study in which pregnant rats were administered piroxicam at 2, 5, or 10 mg/kg/day on Gestation Day 15 through delivery and weaning of offspring, reduced weight gain and death were observed in dams at 10 mg/kg/day (5 times the MRHD based on a mg/m2 BSA) starting on Gestation Day 20. Treated dams revealed peritonitis, adhesions, gastric bleeding, hemorrhagic enteritis and dead fetuses in utero. Parturition was delayed and there was an increased incidence of stillbirth in all piroxicam- treated groups (at doses equivalent to the MRHD). Postnatal development could not be reliably assessed due to the absence of maternal care secondary to severe maternal toxicity.
8.2 Lactation
Risk Summary
Limited data from 2 published reports that included a total of 6 breastfeeding women and 2 infants showed piroxicam is excreted in human milk at approximately 1% to 3% of the maternal concentration. No accumulation of piroxicam occurred in milk relative to that in maternal plasma during treatment. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for piroxicam capsules and any potential adverse effects on the breastfed infant from the piroxicam capsules or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Infertility
Females
Based on the mechanism of action, the use of prostaglandin-mediated NSAIDs, including piroxicam capsules, may delay or prevent rupture of ovarian follicles, which has been associated with reversible infertility in some women. Published animal studies have shown that administration of prostaglandin synthesis inhibitors has the potential to disrupt prostaglandin- mediated follicular rupture required for ovulation. Small studies in women treated with NSAIDs have also shown a reversible delay in ovulation. Consider withdrawal of NSAIDs, including piroxicam capsules, in women who have difficulties conceiving or who are undergoing investigation of infertility.
8.4 Pediatric Use
Piroxicam capsules has not been investigated in pediatric patients. The safety and effectiveness of piroxicam capsules have not been established.
8.5 Geriatric Use
Elderly patients, compared to younger patients, are at greater risk for NSAID- associated serious cardiovascular, gastrointestinal, and/or renal adverse reactions. If the anticipated benefit for the elderly patient outweighs these potential risks, start dosing at the low end of the dosing range, and monitor patients for adverse effects [see Warnings and Precautions (5.1,5.2, 5.3, 5.6, 5.13)].
Pregnancy: Use of NSAIDs during the third trimester of pregnancy increases the risk of premature closure of the fetal ductus arteriosus. Avoid use of NSAIDs in pregnant women starting at 30 weeks gestation (5.10, 8.1)
Infertility: NSAIDs are associated with reversible infertility. Consider withdrawal of piroxicam capsules in women who have difficulties conceiving (8.3)
OVERDOSAGE SECTION
10 OVERDOSAGE
Symptoms following acute NSAID overdoses have been typically limited to lethargy, drowsiness, nausea, vomiting, and epigastric pain, which are generally reversible with supportive care. Gastrointestinal bleeding has occurred. Hypertension, acute renal failure, respiratory depression, and coma have occurred, but were rare [see Warnings and Precautions (5.1, 5.2, 5.4, 5.6)].
Manage patients with symptomatic and supportive care following an NSAID overdose. There are no specific antidotes. Consider emesis and/or activated charcoal (60 grams to 100 grams in adults, 1 gram to 2 grams per kg of body weight in pediatric patients) and/or osmotic cathartic in symptomatic patients seen within four hours of ingestion or in patients with a large overdosage (5 to 10 times the recommended dosage).
The long plasma half-life of piroxicam should be considered when treating an overdose with piroxicam. Forced diuresis, alkalinization of urine, hemodialysis, or hemoperfusion may not be useful due to high protein binding.
For additional information about overdosage treatment contact a poison control center (1-800-222-1222).
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
In controlled clinical trials, the effectiveness of piroxicam capsules has been established for both acute exacerbations and long term management of rheumatoid arthritis and osteoarthritis.
The therapeutic effects of piroxicam capsules are evident early in the treatment of both diseases with a progressive increase in response over several (8-12) weeks. Efficacy is seen in terms of pain relief and, when present, subsidence of inflammation.
Doses of 20 mg/day piroxicam capsules display a therapeutic effect comparable to therapeutic doses of aspirin, with a lower incidence of minor gastrointestinal effects and tinnitus.
Piroxicam capsules have been administered concomitantly with fixed doses of gold and corticosteroids. The existence of a “steroid sparing” effect has not been adequately studied to date.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Piroxicam Capsules, USP
The 10 mg capsules are hard gelatin capsules with a swedish orange opaque cap and ivory opaque body containing white to off-white powder.
NDC: 72162-1290-1: 100 Capsules in a BOTTLE, PLASTIC
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F). [see USP Controlled Room Temperature].
Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
Repackaged/Relabeled by:
Bryant Ranch Prepack, Inc.
Burbank, CA 91504
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide) that accompanies each prescription dispensed. Inform patients, families, or their caregivers of the following information before initiating therapy with piroxicam capsules and periodically during the course of ongoing therapy.
Cardiovascular Thrombotic Events Advise patients to be alert for the symptoms of cardiovascular thrombotic events, including chest pain, shortness of breath, weakness, or slurring of speech, and to report any of these symptoms to their health care provider immediately [see Warnings and Precautions (5.1)].
Gastrointestinal Bleeding, Ulceration, and Perforation Advise patients to report symptoms of ulcerations and bleeding, including epigastric pain, dyspepsia, melena, and hematemesis to their healthcare provider. In the setting of concomitant use of low-dose aspirin for cardiac prophylaxis, inform patients of the increased risk for and the signs and symptoms of GI bleeding [see Warnings and Precautions (5.2)].
HepatotoxicityInform patients of the warning signs and symptoms of hepatotoxicity (e.g., nausea, fatigue, lethargy, pruritus, diarrhea, jaundice, right upper quadrant tenderness, and “flu-like” symptoms). If these occur, instruct patients to stop piroxicam capsules and seek immediate medical therapy [see Warnings and Precautions (5.3)].
Heart Failure and EdemaAdvise patients to be alert for the symptoms of congestive heart failure including shortness of breath, unexplained weight gain, or edema and to contact their healthcare provider if such symptoms occur [see Warnings and Precautions (5.5)].
Anaphylactic ReactionsInform patients of the signs of an anaphylactic reaction (e.g., difficulty breathing, swelling of the face or throat). Instruct patients to seek immediate emergency help if these occur [see Contraindications (4) and Warnings and Precautions (5.7)].
Serious Skin ReactionsAdvise patients to stop piroxicam capsules immediately if they develop any type of rash and to contact their healthcare provider as soon as possible [see Warnings and Precautions (5.9)].
Female FertilityAdvise females of reproductive potential who desire pregnancy that NSAIDs, including piroxicam capsules, may be associated with a reversible delay in ovulation [see Use in Specific Populations (8.3)].
Fetal ToxicityInform pregnant women to avoid use of piroxicam capsules and other NSAIDs starting at 30 weeks gestation because of the risk of the premature closing of the fetal ductus arteriosus [see Warnings and Precautions (5.10) and Use in Specific Populations (8.1)].
Avoid Concomitant Use of NSAIDsInform patients that the concomitant use of piroxicam capsules with other NSAIDs or salicylates (e.g., diflunisal, salsalate) is not recommended due to the increased risk of gastrointestinal toxicity, and little or no increase in efficacy [see Warnings and Precautions (5.2) and Drug Interactions (7)]. Alert patients that NSAIDs may be present in “over the counter” medications for treatment of colds, fever, or insomnia.
Use of NSAIDs and Low-Dose AspirinInform patients not to use low-dose aspirin concomitantly with piroxicam capsules until they talk to their healthcare provider [see Drug Interactions (7)].
Manufactured by:
Nostrum Laboratories, Inc.
Kansas City, MO 64120
Rev: November 2019
SPL MEDGUIDE SECTION
Medication Guide for Nonsteroidal Anti-inflammatory Drugs (NSAIDs)
|
What is the most important information I should know about medicines called Nonsteroidal Anti-inflammatory Drugs (NSAIDs)? NSAIDs can cause serious side effects, including: • o o Do not take NSAIDs right before or after a heart surgery called a “coronary artery bypass graft (CABG).” Avoid taking NSAIDs after a recent heart attack, unless your healthcare provider tells you to. You may have an increased risk of another heart attack if you take NSAIDs after a recent heart attack. • o o o The risk of getting an ulcer or bleeding increases with: o o | |
|
o o o o |
o o o o |
|
NSAIDs should only be used: o o o | |
|
What are NSAIDs? NSAIDs are used to treat pain and redness, swelling, and heat (inflammation) from medical conditions such as different types of arthritis, menstrual cramps, and other types of short-term pain. | |
|
Who should not take NSAIDs? Do not take NSAIDs: • • | |
|
Before taking NSAIDs, tell your healthcare provider about all of your medical conditions, including if you: • • • • • **Tell your healthcare provider about all of the medicines you take, including prescription or over-the-counter medicines, vitamins or herbal supplements. **NSAIDs and some other medicines can interact with each other and cause serious side effects.Do not start taking any new medicine without talking to your healthcare provider first. | |
|
What are the possible side effects of NSAIDs? NSAIDs can cause serious side effects, including: See “What is the most important information I should know about medicines called Nonsteroidal Anti-inflammatory Drugs (NSAIDs)?” • • • • • • • • Get emergency help right away if you get any of the following symptoms: | |
|
• • • |
• • |
|
Stop taking your NSAID and call your healthcare provider right away if you get any of the following symptoms: | |
|
• • • • • • • |
• • • • • |
|
If you take too much of your NSAID, call your healthcare provider or get medical help right away. These are not all the possible side effects of NSAIDs. For more information, ask your healthcare provider or pharmacist about NSAIDs. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. | |
|
Other information about NSAIDs • • | |
|
General information about the safe and effective use of NSAIDs Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use NSAIDs for a condition for which they were not prescribed. Do not give NSAIDs to other people, even if they have the same symptoms that you have. It may harm them. If you would like more information about NSAIDs, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about NSAIDs that is written for health professionals. This Medication Guide has been approved by the U.S. Food and Drug Administration. For more information, please contact Nostrum Laboratories, Inc. at quality@nostrumpharma.com at 1-877-770-1288 |
Manufactured by:
Nostrum Laboratories, Inc.
Kansas City, MO 64120
Rev: November 2019
DESCRIPTION SECTION
11 DESCRIPTION
Piroxicam Capsules, USP is a nonsteroidal anti-inflammatory drug, available as Ivory opaque/Swedish orange opaque 10 mg capsules and Swedish orange opaque/Swedish orange opaque 20 mg capsules for oral administration. The chemical name is 4-hydroxyl-2-methyl-N-2-pyridinyl-2H-1,2,-benzothiazine-3-carboxamide 1,1-dioxide. The molecular weight is 331.35. Its molecular formula is C15H13N3O4S, and it has the following chemical structure.
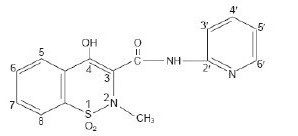
Piroxicam occurs as a white crystalline solid, sparingly soluble in water, dilute acid, and most organic solvents. It is slightly soluble in alcohol and in aqueous solutions. It exhibits a weakly acidic 4-hydroxy proton (pKa 5.1) and a weakly basic pyridyl nitrogen (pKa 1.8).
The inactive ingredients in piroxicam capsules include: Hard gelatin capsules (which may contain FD&C Blue 1, FD&C Red 40, D&C Yellow 10, titanium dioxide and gelatin inactive ingredients), corn starch, lactose, magnesium stearate and sodium lauryl sulfate.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Piroxicam has analgesic, anti-inflammatory, and antipyretic properties.
The mechanism of action of piroxicam capsules, like that of other NSAIDs, is not completely understood but involves inhibition of cyclooxygenase (COX-1 and COX-2).
Piroxicam is a potent inhibitor of prostaglandin (PG) synthesis in vitro. Piroxicam concentrations reached during therapy have produced in vivo effects. Prostaglandins sensitize afferent nerves and potentiate the action of bradykinin in inducing pain in animal models. Prostaglandins are mediators of inflammation. Because piroxicam is an inhibitor of prostaglandin synthesis, its mode of action may be due to a decrease of prostaglandins in peripheral tissues.
12.3 Pharmacokinetics
General Pharmacokinetic Characteristics
The pharmacokinetics of piroxicam have been characterized in healthy subjects, special populations and patients. The pharmacokinetics of piroxicam are linear. Proportional increase in exposure is observed with increasing doses. The prolonged half-life (50 hours) results in the maintenance of relatively stable plasma concentrations throughout the day on once daily doses and significant accumulation upon multiple dosing. Most patients approximate steady state plasma levels within 7 to 12 days. Higher levels, which approximate steady state at two to three weeks, have been observed in patients in whom longer plasma half-lives of piroxicam occurred.
Absorption
Piroxicam is well absorbed following oral administration. Drug plasma concentrations are proportional for 10 mg and 20 mg doses and generally peak within three to five hours after administration. A single 20 mg dose generally produces peak piroxicam plasma levels of 1.5 mcg/mL to 2 mcg/mL, while maximum drug plasma concentrations, after repeated daily administration of 20 mg piroxicam, usually stabilize at 3 mcg/mL to 8 mcg/mL.
With food there is a slight delay in the rate but not the extent of absorption following oral administration. The concomitant administration of antacids (aluminum hydroxide or aluminum hydroxide with magnesium hydroxide) have been shown to have no effect on the plasma levels of orally administered piroxicam.
Distribution
The apparent volume of distribution of piroxicam is approximately 0.14 L/kg. Ninety nine percent of plasma piroxicam is bound to plasma proteins. Piroxicam is excreted into human milk. The presence in breast milk has been determined during initial and long term conditions (52 days). Piroxicam appeared in breast milk at approximately 1% to 3% of the maternal concentration. No accumulation of piroxicam occurred in milk relative to that in plasma during treatment.
Elimination
Metabolism
Metabolism of piroxicam occurs by hydroxylation at the 5 position of the pyridyl side chain and conjugation of this product; by cyclodehydration; and by a sequence of reactions involving hydrolysis of the amide linkage, decarboxylation, ring contraction, and N-demethylation. In vitro studies indicate cytochrome P4502C9 (CYP2C9) as the main enzyme involved in the formation to the 5′-hydroxy-piroxicam, the major metabolite [see Clinical Pharmacology (12.5)]. The biotransformation products of piroxicam metabolism are reported to not have any anti-inflammatory activity.
Higher systemic exposure of piroxicam has been noted in subjects with CYP2C9 polymorphisms compared to normal metabolizer type subjects [see Clinical Pharmacology (12.5)].
Excretion
Piroxicam and its biotransformation products are excreted in urine and feces, with about twice as much appearing in the urine as in the feces. Approximately 5% of a piroxicam capsule dose is excreted unchanged. The plasma half-life (t½) for piroxicam is approximately 50 hours.
Specific Populations
Pediatric
Piroxicam has not been investigated in pediatric patients.
Race
Pharmacokinetic differences due to race have not been identified.
Hepatic Impairment
The effects of hepatic disease on piroxicam pharmacokinetics have not been established. However, a substantial portion of piroxicam elimination occurs by hepatic metabolism. Consequently, patients with hepatic disease may require reduced doses of piroxicam as compared to patients with normal hepatic function.
Renal Impairment
Piroxicam pharmacokinetics have been investigated in patients with renal insufficiency. Studies indicate patients with mild to moderate renal impairment may not require dosing adjustments. However, the pharmacokinetic properties of piroxicam in patients with severe renal insufficiency or those receiving hemodialysis are not known.
Drug Interaction Studies
Antacids
Concomitant administration of antacids had no effect on piroxicam plasma levels.
Aspirin
When piroxicam was administered with aspirin, its protein binding was reduced, although the clearance of free piroxicam capsules was not altered. Plasma levels of piroxicam were decreased to approximately 80% of their normal values when piroxicam capsules was administered (20 mg/day) in conjunction with aspirin (3900 mg/day). The clinical significance of this interaction is not known [see Drug Interactions (7)].
12.5 Pharmacogenomics
CYP2C9 activity is reduced in individuals with genetic polymorphisms, such as the CYP2C92 and CYP2C93 polymorphisms. Limited data from two published reports showed that subjects with heterozygous CYP2C9*1/2 (n = 9), heterozygous CYP2C91/3 (n = 9), and homozygous CYP2C93/3 (n = 1) genotypes showed 1.7-, 1.7-, and 5.3-fold higher piroxicam systemic levels, respectively, than the subjects with CYP2C91/1 (n = 17, normal metabolizer genotype) following administration of a single oral dose. The mean elimination half-life values of piroxicam for subjects with CYP2C91/3 (n = 9) and CYP2C93/3 (n = 1) genotypes were 1.7- and 8.8-fold higher than subjects with CYP2C91/1 (n = 17). It is estimated that the frequency of the homozygous3/*3 genotype is 0% to 1% in the population at large; however, frequencies as high as 5.7% have been reported in certain ethnic groups.
Poor Metabolizers of CYP2C9 Substrates: In patients who are known or
suspected to be poor CYP2C9 metabolizers based on genotype or previous history/experience with other CYP2C9 substrates (such as warfarin and phenytoin) consider dose reduction as they may have abnormally high plasma levels due to reduced metabolic clearance.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term animal studies have not been conducted to characterize the carcinogenic potential of piroxicam.
Mutagenesis
Piroxicam was not mutagenic in an Ames bacterial reverse mutation assay, or in a dominant lethal mutation assay in mice, and was not clastogenic in an in vivo chromosome aberration assay in mice.
Impairment of Fertility
Reproductive studies in which rats were administered piroxicam at doses of 2, 5, or 10 mg/kg/day (up to 5 times the MRHD of 20 mg based on mg/m2 body surface area [BSA]) revealed no impairment of male or female fertility.
