Visudyne
These highlights do not include all the information needed to use VISUDYNE safely and effectively. See full prescribing information for VISUDYNE. VISUDYNE (verteporfin for injection), for intravenous use Initial U.S. Approval: 2000
952f4c80-50b1-4308-9ee6-311ffefb13df
HUMAN PRESCRIPTION DRUG LABEL
Aug 27, 2025
Bausch Health US LLC
DUNS: 831922468
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
verteporfin for injection
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC0187-5600-15
Rx only
Visudyne**®**
(verteporfin
for injection)
Sterile
15 mg
1 Single-Use Vial
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
VISUDYNE ®(verteporfin for injection) therapy is indicated for the treatment of patients with predominantly classic subfoveal choroidal neovascularization (CNV) due to age-related macular degeneration (AMD), pathologic myopia or presumed ocular histoplasmosis.
There is insufficient evidence to indicate VISUDYNE for the treatment of predominantly occult subfoveal CNV.
VISUDYNE (verteporfin for injection) therapy is a photoenhancer indicated for the treatment of patients with predominantly classic subfoveal choroidal neovascularization due to age-related macular degeneration, pathologic myopia or presumed ocular histoplasmosis. ( 1)
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
VISUDYNE (verteporfin for injection) is contraindicated for patients with porphyria or a known hypersensitivity to any component of this preparation [see Adverse Reactions( 6)] .
VISUDYNE (verteporfin for injection) is contraindicated for patients with porphyria or a known hypersensitivity to any component of this preparation. ( 4)
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Local Adverse Reactions - Extravasation
Standard precautions should be taken during infusion of VISUDYNE (verteporfin for injection) to avoid extravasation. Examples of standard precautions include, but are not limited to:
- A free-flowing intravenous (IV) line should be established before starting VISUDYNE infusion and the line should be carefully monitored.
- Due to the possible fragility of vein walls of some elderly patients, it is strongly recommended that the largest arm vein possible, preferably antecubital, be used for injection.
- Small veins in the back of the hand should be avoided.
Extravasation of VISUDYNE, especially if the affected area is exposed to light, can cause severe pain, inflammation, swelling or discoloration at the injection site. Localized (skin) necrosis at the injection site following extravasation has also been reported.
If extravasation does occur, the infusion should be stopped immediately. The extravasation area must be thoroughly protected from direct light until swelling and discoloration have faded in order to prevent the occurrence of local burn, which could be severe. Cold compresses should be applied to the injection site. Oral medications for pain relief may be administered.
5.2 Exposure to Sun or Direct Light
Following injection with VISUDYNE (verteporfin for injection), care should be taken to avoid exposure of skin or eyes to direct sunlight or bright indoor light for 5 days. In the event of extravasation during infusion, the extravasation area must be thoroughly protected from direct light until the swelling and discoloration have faded in order to prevent the occurrence of a local burn which could be severe. If emergency surgery is necessary within 48 hours after treatment, as much of the internal tissue as possible should be protected from intense light.
5.3 Decreased Vision After Treatment
Patients who experience severe decrease of vision of 4 lines or more within 1 week after treatment should not be retreated, at least until their vision completely recovers to pretreatment levels and the potential benefits and risks of subsequent treatment are carefully considered by the treating physician.
5.4 Anaphylactic Reactions
Cases of anaphylactic reactions have been reported in patients receiving VISUDYNE. Medical supervision is recommended during infusion. If an anaphylactic or other serious allergic reaction occurs during or following infusion, administration of VISUDYNE should be discontinued immediately and appropriate therapy should be initiated.
- Extravasation: If extravasation occurs, the infusion should be stopped immediately. The extravasation area must be thoroughly protected from direct light until swelling and discoloration have faded in order to prevent the occurrence of local burn. ( 5.1)
- Exposure to Sun or Direct Light: Following injection with VISUDYNE (verteporfin for injection), care should be taken to avoid exposure of skin or eyes to direct sunlight or bright indoor light for 5 days. ( 5.2)
- Anaphylactic Reactions: Immediately discontinue administration of VISUDYNE and initiate appropriate therapy if an anaphylactic or other serious allergic reaction occurs during or following infusion. ( 5.4)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- Local Adverse Reactions – Extravasation [see Warnings and Precautions( 5.1)]
- Exposure to Sun or Direct Light [see Warnings and Precautions( 5.2)]
- Decreased Vision after Treatment [see Warnings and Precautions( 5.3)]
- Porphyria and Hypersensitivity [see Contraindications( 4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Severe chest pain, vasovagal and hypersensitivity reactions have been reported. Vasovagal and hypersensitivity reactions on rare occasions can be severe. These reactions may include syncope, sweating, dizziness, rash, dyspnea, flushing and changes in blood pressure and heart rate. General symptoms can include headache, malaise, urticaria, and pruritus.
The most frequently reported adverse reactions to VISUDYNE (verteporfin for injection) are injection site reactions (including pain, edema, inflammation, extravasation, rashes, hemorrhage and discoloration) and visual disturbances (including blurred vision, flashes of light, decreased visual acuity and visual field defects, including scotoma). These events occurred in approximately 10%-30% of patients. The following events, listed by Body System, were reported more frequently with VISUDYNE therapy than with placebo therapy and occurred in 1%-10% of patients:
Ocular Treatment Site: Blepharitis, cataracts, conjunctivitis/conjunctival
injection, dry eyes, ocular
itching, severe vision decrease with or without subretinal/retinal or vitreous
hemorrhage
Body as a Whole: Asthenia, fever, flu syndrome, infusion related pain
primarily presenting as
back pain, photosensitivity reactions
Cardiovascular: Atrial fibrillation, hypertension, peripheral vascular disorder, varicose veins
Dermatologic: Eczema
Digestive: Constipation, gastrointestinal cancers, nausea
Hemic and Lymphatic: Anemia, white blood cell count decreased, white blood cell count increased
Hepatic: Elevated liver function tests
Metabolic/Nutritional: Albuminuria, creatinine increased
Musculoskeletal: Arthralgia, arthrosis, myasthenia
Nervous System: Hypesthesia, sleep disorder, vertigo
Respiratory: Cough, pharyngitis, pneumonia
Special Senses: Cataracts, decreased hearing, diplopia, lacrimation disorder
Urogenital: Prostatic disorder
Severe vision decrease, equivalent of >4 lines, within 7 days after treatment has been reported in 1%-5% of patients. Partial recovery of vision was observed in some patients. Photosensitivity reactions usually occurred in the form of skin sunburn following exposure to sunlight. The higher incidence of back pain in the VISUDYNE group occurred primarily during infusion.
The following adverse events have occurred either at low incidence (<1%) during clinical trials or have been reported during the use of VISUDYNE in clinical practice where these reactions were reported voluntarily from a population of unknown size and frequency of occurrence cannot be determined precisely. They have been chosen for inclusion based on factors such as seriousness, frequency of reporting, possible causal connection to VISUDYNE, or a combination of these factors:
Ocular Treatment Site: Retinal detachment (nonrhegmatogenous), retinal or
choroidal vessel
nonperfusion, retinal pigment epithelial tear.
Non-ocular Events: Chest pain and other musculoskeletal pain during infusion,
anaphylactic
reaction during or following infusion, injection site necrosis.
Most common adverse reactions (incidence ˃10%) are injection site reactions and visual disturbances. ( 6.1)
**To report SUSPECTED ADVERSE REACTIONS, contact Bausch & Lomb Incorporated at 1-800-321-4576 or FDA at 1-800-FDA-1088 or **www.fda.gov/medwatch.
RECENT MAJOR CHANGES SECTION
RECENT MAJOR CHANGES
Warnings and Precautions ( 5.1, 5.4) 7/2021
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
A course of VISUDYNE (verteporfin for injection) therapy is a two-step process requiring administration of both drug and light.
- The first step is the administration of VISUDYNE.
- The second step is the activation of VISUDYNE with light from a nonthermal diode laser.
The physician should re-evaluate the patient 3 months after treatment and if choroidal neovascular leakage is detected on fluorescein angiography, therapy may be repeated.
Lesion Size Determination
The greatest linear dimension (GLD) of the lesion should be estimated by fluorescein angiography and color fundus photography. All classic and occult CNV, blood and/or blocked fluorescence, and any serous detachments of the retinal pigment epithelium should be included for this measurement. Fundus cameras with magnification within the range of 2.4-2.6X are recommended. The GLD of the lesion on the fluorescein angiogram must be corrected for the magnification of the fundus camera to obtain the GLD of the lesion on the retina.
Spot Size Determination
The treatment spot size should be 1,000 microns larger than the GLD of the lesion on the retina to allow a 500 micron border, ensuring full coverage of the lesion. The maximum spot size used in the clinical trials was 6,400 microns.
The nasal edge of the treatment spot must be positioned at least 200 microns from the temporal edge of the optic disc, even if this will result in lack of photoactivation of CNV within 200 microns of the optic nerve.
2.2 Recommended Dosage of VISUDYNE
The recommended dose of VISUDYNE is 6 mg/m 2body surface area.
2.3 VISUDYNE Administration
Avoid contact with the eyes and skin during preparation and administration of VISUDYNE. Because of the potential to induce photosensitivity reactions, any exposed person must be protected from bright light [see Warnings and Precautions( 5.1) and How Supplied/Storage and Handling( 16)] .
Reconstitution
Reconstitute each vial of VISUDYNE with 7 mL of Sterile Water for Injection. Each reconstituted vial provides 7.5 mL solution containing 2 mg/mL of verteporfin. Reconstituted VISUDYNE must be protected from light and used within 4 hours. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Reconstituted VISUDYNE is an opaque dark green solution.
Dilution
VISUDYNE may precipitate in saline solutions. Do not use normal saline or other parenteral solutions, except 5% Dextrose for Injection, for dilution of the reconstituted VISUDYNE. Do not mix VISUDYNE in the same solution with other drugs.
The volume of reconstituted VISUDYNE required to achieve the desired dose of 6 mg/m 2body surface area is withdrawn from the vial and diluted with 5% Dextrose for Injection to a total infusion volume of 30 mL. After dilution, protect from light and use within 4 hours.
Intravenous Infusion
The full infusion volume is administered intravenously over 10 minutes at a rate of 3 mL/minute, using an appropriate syringe pump and in-line filter. The clinical studies were conducted using a standard infusion line filter of 1.2 microns.
Precautions should be taken to prevent extravasation at the injection site. If extravasation occurs, protect the site from light [see Warnings and Precautions( 5.1)] .
2.4 Light Administration
Initiate 689 nm wavelength laser light delivery to the patient 15 minutes after the start of the 10-minute infusion with VISUDYNE.
Photoactivation of VISUDYNE is controlled by the total light dose delivered. In the treatment of CNV, the recommended light dose is 50 J/cm 2of neovascular lesion administered at an intensity of 600 mW/cm 2. This dose is administered over 83 seconds.
Light dose, light intensity, ophthalmic lens magnification factor and zoom lens setting are important parameters for the appropriate delivery of light to the predetermined treatment spot. Follow the laser system manuals for procedure set up and operations.
The laser system must deliver a stable power output at a wavelength of 689±3 nm. Light is delivered to the retina as a single circular spot via a fiber optic and a slit lamp, using a suitable ophthalmic magnification lens.
The following laser systems have been tested for compatibility with VISUDYNE and are approved for delivery of a stable power output at a wavelength of 689±3 nm:
- Coherent Opal Photoactivator laser console and modified Coherent LaserLink adapter, manufactured by Lumenis, Inc., 2400 Condensa Street, Santa Clara, CA 95051-0901,
- Zeiss VISULAS 690s laser and VISULINK PDT adapter manufactured by Carl Zeiss Meditec Inc., 5160 Hacienda Drive, Dublin, CA 94568,
- Ceralas I laser system and Ceralink Slit Lamp Adapter manufactured by Biolitec Inc., 515 Shaker Road, East Longmeadow, MA 01028,
- Quantel Activis laser console and the ZSL30 ACT, ZSL120 ACT and HSBMBQ ACT slit lamp adapters distributed by Quantel Medical, 601 Haggerty Lane, Bozeman, MT 59715.
2.5 Concurrent Bilateral Treatment
The controlled trials only allowed treatment of one eye per patient. In patients who present with eligible lesions in both eyes, physicians should evaluate the potential benefits and risks of treating both eyes concurrently. If the patient has already received previous VISUDYNE therapy in one eye with an acceptable safety profile, both eyes can be treated concurrently after a single administration of VISUDYNE. The more aggressive lesion should be treated first, at 15 minutes after the start of infusion. Immediately at the end of light application to the first eye, the laser settings should be adjusted to introduce the treatment parameters for the second eye, with the same light dose and intensity as for the first eye, starting no later than 20 minutes from the start of infusion.
In patients who present for the first time with eligible lesions in both eyes without prior VISUDYNE therapy, it is prudent to treat only one eye (the most aggressive lesion) at the first course. One week after the first course, if no significant safety issues are identified, the second eye can be treated using the same treatment regimen after a second VISUDYNE infusion. Approximately 3 months later, both eyes can be evaluated and concurrent treatment following a new VISUDYNE infusion can be started if both lesions still show evidence of leakage.
- Recommended Dose: 6 mg/m 2body surface area. ( 2.2)
- Reconstitution: Reconstitute each vial of VISUDYNE with 7 mL of Sterile Water for Injection to provide 7.5 mL containing 2 mg/mL of verteporfin. Reconstituted VISUDYNE must be protected from light and used within 4 hours. ( 2.3)
- Dilution: Dilute desired dose of reconstituted VISUDYNE with 5% Dextrose for Injection to a total infusion volume of 30 mL. ( 2.3)
- Infusion: Administer intravenously over 10 minutes at a rate of 3 mL/minute, using an appropriate syringe pump and in-line filter. ( 2.3)
- Light Administration: The recommended light dose is 50 J/cm 2of neovascular lesion administered at an intensity of 600 mW/cm 2. The wavelength of the laser light should be 689±3 nm. This light dose is administered over 83 seconds, starting 15 minutes after the start of the VISUDYNE infusion. ( 2.4)
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
For injection: 15 mg of verteporfin as a dark green lyophilized cake in a single-dose vial for reconstitution. VISUDYNE is intended for intravenous injection only. Each reconstituted vial provides 7.5 mL solution containing 2 mg/mL of verteporfin.
- For injection: 15 mg of verteporfin as a dark green lyophilized cake in a single-dose vial for reconstitution. ( 3)
- Each reconstituted vial provides 7.5 mL solution containing 2 mg/mL of verteporfin. ( 3)
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no data with the use of VISUDYNE in pregnant women to inform a drug- associated risk. Intravenous administration of verteporfin to pregnant rats during the period of organogenesis produced an increase in the incidence of anophthalmia/microphthalmia and wavy ribs at exposures approximately 40-fold the human exposure at the recommended clinical dose.
Verteporfin did not produce adverse fetal effect in rats or rabbits at exposures 6- to 20-fold the human exposure at the recommended clinical dose.
There are no adequate and well-controlled studies in pregnant women. VISUDYNE should be used during pregnancy only if the benefit justifies the potential risk to the fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2%-4% and 15%-20%, respectively.
Data
Animal Data
Rat fetuses of dams administered verteporfin for injection intravenously during organogenesis exhibited an increase in the incidence of anophthalmia/microphthalmia and wavy ribs at doses ≥10 mg/kg/day (approximately 40-fold the human exposure at the recommended dose of 6 mg/m 2, based on AUC in female rats). No teratogenic effects were observed in rat fetuses at a dose of 2 mg/kg/day (approximately 6-fold the human exposure at the recommended dose of 6 mg/m 2, based on AUC in female rats).
In pregnant rabbits, a decrease in maternal body weight gain and food consumption was observed in animals that received verteporfin for injection intravenously at doses up to 10 mg/kg/day during organogenesis. The no observed adverse effect level (NOAEL) for maternal toxicity was 3 mg/kg/day (approximately 6-fold the recommended human dose of 6 mg/m 2, based on body surface area). No teratogenic effects were observed in rabbit fetuses at doses up to 10 mg/kg/day (approximately 20-fold the recommended human dose of 6 mg/m 2, based on body surface area).
8.2 Lactation
Risk Summary
Verteporfin and its diacid metabolite have been found in human breast milk following an intravenous infusion at the recommended human dose of 6 mg/m 2. Verteporfin was present in breast milk at levels up to 66% of the corresponding plasma levels and declined below the limit of quantification (2 ng/mL) within 24 hours. The diacid metabolite had lower peak concentrations but persisted up to at least 48 hours.
Because of the potential for serious adverse reactions in nursing infants from VISUDYNE, a decision should be made whether to discontinue nursing or postpone treatment, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
8.5 Geriatric Use
Approximately 90% of the patients treated with VISUDYNE in the clinical efficacy trials were over the age of 65. A reduced treatment effect was seen with increasing age.
DESCRIPTION SECTION
11 DESCRIPTION
VISUDYNE (verteporfin for injection) is a light activated drug used in photodynamic therapy. The finished drug product is a lyophilized dark green cake. Verteporfin is a 1:1 mixture of two regioisomers (I and II), represented by the following structures:
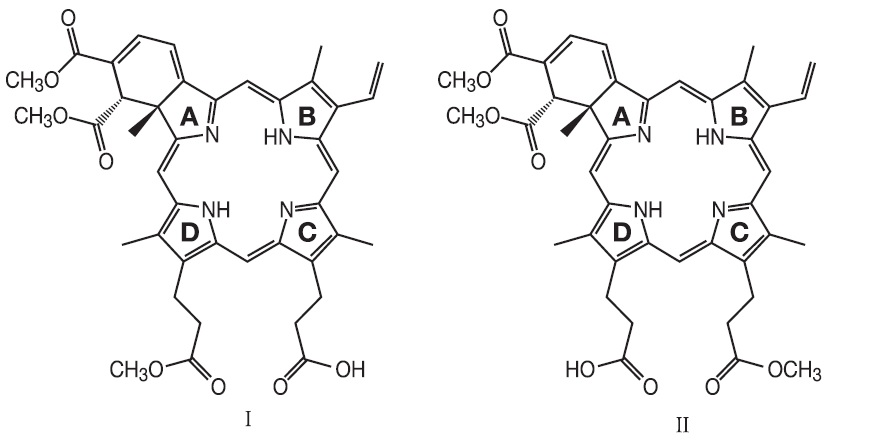
The chemical names for the verteporfin regioisomers are:
9-methyl (I) and 13-methyl (II) trans-(±)-18-ethenyl-4,4a,-dihydro-3,4-bis(methoxycarbonyl)-4a,8,14,19-tetramethyl-23H, 25H-benzo[b]porphine-9,13-dipropanoate
The molecular formula is C 41H 42N 4O 8with a molecular weight of approximately 718.8. Each mL of reconstituted VISUDYNE contains:
- ACTIVE: verteporfin, 2 mg
- INACTIVES: ascorbyl palmitate, butylated hydroxytoluene, dimyristoyl phosphatidylcholine, egg phosphatidylglycerol and lactose
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
VISUDYNE (verteporfin for injection) therapy is a two-stage process requiring administration of both verteporfin for injection and nonthermal red light.
Verteporfin is transported in the plasma primarily by lipoproteins. Once verteporfin is activated by light in the presence of oxygen, highly reactive, short-lived singlet oxygen and reactive oxygen radicals are generated. Light activation of verteporfin results in local damage to neovascular endothelium, resulting in vessel occlusion. Damaged endothelium is known to release procoagulant and vasoactive factors through the lipoxygenase (leukotriene) and cyclooxygenase (eicosanoids such as thromboxane) pathways, resulting in platelet aggregation, fibrin clot formation and vasoconstriction. Verteporfin appears to somewhat preferentially accumulate in neovasculature, including choroidal neovasculature. However, animal models indicate that the drug is also present in the retina. Therefore, there may be collateral damage to retinal structures following photoactivation including the retinal pigmented epithelium and outer nuclear layer of the retina. The temporary occlusion of the CNV following VISUDYNE therapy has been confirmed in humans by fluorescein angiography.
12.3 Pharmacokinetics
Following intravenous infusion, verteporfin exhibits a bi-exponential elimination with a terminal elimination half-life of approximately 5-6 hours. The extent of exposure and the maximal plasma concentration are proportional to the dose between 6 and 20 mg/m 2. At the intended dose, pharmacokinetic parameters are not significantly affected by gender.
Verteporfin is metabolized to a small extent to its diacid metabolite by liver and plasma esterases. NADPH-dependent liver enzyme systems (including the cytochrome P450 isozymes) do not appear to play a role in the metabolism of verteporfin. Elimination is by the fecal route, with less than 0.01% of the dose recovered in urine.
In a study of patients with mild hepatic insufficiency (defined as having two abnormal hepatic function tests at enrollment), AUC and C maxwere not significantly different from the control group; half-life, however, was significantly increased by approximately 20%.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
No studies have been conducted to determine the carcinogenic potential of
verteporfin.
Mutagenesis
Photodynamic therapy (PDT) as a class has been reported to result in DNA
damage including DNA strand breaks, alkali-labile sites, DNA degradation, and
DNA-protein cross-links which may result in chromosomal aberrations, sister
chromatid exchanges (SCE), and mutations. In addition, other photodynamic
therapeutic agents have been shown to increase the incidence of SCE in Chinese
hamster ovary (CHO) cells irradiated with visible light and in Chinese hamster
lung fibroblasts irradiated with near UV light, increase mutations and DNA-
protein cross-linking in mouse L5178 cells, and increase DNA-strand breaks in
malignant human cervical carcinoma cells, but not in normal cells. Verteporfin
was not evaluated in these latter systems. It is not known how the potential
for DNA damage with PDT agents translates into human risk.
Impairment of Fertility
No effect on male or female fertility has been observed in rats following
intravenous administration of verteporfin for injection up to 10 mg/kg/day
(approximately 60- and 40-fold human exposure at 6 mg/m 2based on AUC in male
and female rats, respectively).
13.2 Animal Toxicology and/or Pharmacology
At a >10-fold higher dose given by bolus injection to sedated or anesthetized pigs, verteporfin caused severe hemodynamic effects, including death, probably as a result of complement activation. These effects were diminished or abolished by pretreatment with antihistamine and they were not seen in conscious nonsedated pigs.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
VISUDYNE (verteporfin for injection) is supplied in a single-dose glass vial with a gray bromobutyl stopper and aluminum flip-off cap. It contains a lyophilized dark green cake with 15 mg verteporfin.
- NDC 0187-5600-15
Store VISUDYNE between 20°C to 25°C (68°F to 77°F).
16.1 Spills and Disposal
Spills of VISUDYNE should be wiped up with a damp cloth. Skin and eye contact should be avoided due to the potential for photosensitivity reactions upon exposure to light. Use of rubber gloves and eye protection is recommended. All materials should be disposed of properly.
16.2 Accidental Exposure
Because of the potential to induce photosensitivity reactions, it is important to avoid contact with the eyes and skin during preparation and administration of VISUDYNE. Any exposed person must be protected from bright light [see Warnings and Precautions( 5.1)] .
