Ovace
Ovace Plus (sodium sulfacetamide 9.8%) Lotion
073a44cd-f679-48bd-b90d-cf06f32ff07d
HUMAN PRESCRIPTION DRUG LABEL
Aug 13, 2025
Mission Pharmacal Company
DUNS: 008117095
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
SULFACETAMIDE SODIUM
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (18)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL

DESCRIPTION SECTION
DESCRIPTION:
Each gram contains 98 mg of sodium sulfacetamide in a vehicle consisting of: benzyl alcohol, cetearyl alcohol (and) PEG-3 distearoylamidoethylmonium methosulfate (and) polysorbate 60, cetyl alcohol, disodium EDTA, fragrance, glyceryl stearate (and) PEG-100 stearate, magnesium aluminum silicate, PEG-150 distearate, phenoxyethanol, polyethylene glycol 400, purified water, sodium lauryl sulfate, sodium thiosulfate, stearyl alcohol and xanthan gum.
Sodium sulfacetamide is a sulfonamide with antibacterial activity. Sodium sulfacetamide is C 8H 9N 2NaO 3S·H 2O with molecular weight of 254.24. Chemically, sodium sulfacetamide is N-[(4-aminophenyl) sulfonyl]-acetamide, monosodium salt, monohydrate. The structural formula is:
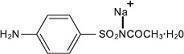
Sodium sulfacetamide is an odorless, white, crystalline powder with a bitter taste. It is freely soluble in water, sparingly soluble in alcohol, while practically insoluble in benzene, in chloroform and in ether.
