Litfulo
These highlights do not include all the information needed to use LITFULO safely and effectively. See full prescribing information for LITFULO. LITFULO™ (ritlecitinib) capsules, for oral use Initial U.S. Approval: 2023
6b2f9446-fb23-4741-b73a-5a2f993733c3
HUMAN PRESCRIPTION DRUG LABEL
Dec 4, 2023
Pfizer Laboratories Div Pfizer Inc
DUNS: 134489525
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
ritlecitinib
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 50 mg Capsule Bottle Label
ALWAYS DISPENSE WITH MEDICATION GUIDE
Pfizer
NDC 0069-0334-28
Litfulo™
(ritlecitinib) capsules
50 mg*
Do not crush, split, or chew
the capsules.
Do not eat the desiccant.
28 Capsules Rx only
SPL UNCLASSIFIED SECTION
For Medical Information about LITFULO, please visit www.pfizermedinfo.com or call 1-800-438-1985.

LAB-1469-1.0
SPL MEDGUIDE SECTION
|
This Medication Guide has been approved by the U.S. Food and Drug Administration. |
Issued: 6/2023 | ||
|
Medication Guide | |||
|
What is the most important information I should know about LITFULO? LITFULO may cause serious side effects, including: 1. Serious infections LITFULO is a medicine that affects your immune system. LITFULO can lower the ability of your immune system to fight infections. Some people have had serious infections while taking LITFULO or other similar medicines, including tuberculosis (TB), and infections caused by bacteria, fungi, or viruses that can spread throughout the body and have been hospitalized. Some people taking similar medicines to LITFULO have died from these infections. • • You should not start taking LITFULO if you have any kind of infection unless your healthcare provider tells you it is okay. You may be at a higher risk of developing shingles (herpes zoster). Before starting LITFULO, tell your healthcare provider if you: • • • • • • • • | |||
|
o o o |
o o o |
o o o | |
|
After starting LITFULO, call your healthcare provider right away if you have any symptoms of an infection. LITFULO can make you more likely to get infections or make any infections that you have worse. If you get a serious infection, your healthcare provider may stop treatment with LITFULO until your infection is controlled. 2. Increased risk of death in people 50 years of age and older who have at least 1 heart disease (cardiovascular) risk factor and are taking a medicine in the class of medicines called Janus kinase (JAK) inhibitors. LITFULO is a kinase inhibitor medicine. 3. Cancer and immune system problems LITFULO may increase your risk of certain cancers by changing the way your immune system works. • • • Tell your healthcare provider if you have ever had any type of cancer. 4. Increased risk of major cardiovascular events such as heart attack, stroke, or death in people 50 years of age and older who have at least 1 heart disease (cardiovascular) risk factor and taking a medicine in the class of medicines called JAK inhibitors, especially if you are a current or past smoker. Get emergency help right awayif you have any symptoms of a heart attack or stroke while using LITFULO, including: • • • • • • • • • 5. Blood clots Blood clots in the veins of your legs (deep vein thrombosis, DVT), lungs (pulmonary embolism, PE), or eyes can happen in some people taking LITFULO. This may be life-threatening. Blood clots in the veins of the legs (deep vein thrombosis, DVT) and lungs (pulmonary embolism, PE) have happened more often in people who are 50 years of age and older with at least 1 heart disease (cardiovascular) risk factor taking a medicine in the class of medicines called JAK inhibitors. • • o o o o 6. Allergic reactions Symptoms that may mean you are having an allergic reaction have been seen during treatment with LITFULO. Some of these reactions were serious. Stop taking LITFULO and get emergency medical help right away if you have symptoms of allergic reaction, including: | |||
|
• • • |
• • | ||
|
7. Changes in certain laboratory test results Your healthcare provider should do blood tests before you start taking LITFULO and during treatment to check for the following: • • • • You should not take LITFULO if your lymphocyte counts or platelet counts are too low or your liver tests are too high. Your healthcare provider may stop your LITFULO treatment for a period of time if needed because of changes in these blood test results. See**"What are the possible side effects of LITFULO?"** for more information about side effects. | |||
|
What is LITFULO? LITFULO is a prescription medicine that is a kinase inhibitor. LITFULO is used to treat an immune system problem that causes severe hair loss (alopecia areata) in adults and children 12 years and older. It is not known if LITFULO is safe and effective in children under 12 years of age. | |||
|
Before taking LITFULO, tell your healthcare provider about all of your medical conditions, including if you: • • • • • • • • Females who are able to become pregnant: o o • Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. LITFULO and other medicines may affect each other causing side effects. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist whenever you get a new medicine. | |||
|
How should I take LITFULO? • • • • If you take too much LITFULO, call the Poison Control Center at 1-800-222-1222 or go to the nearest hospital emergency room right away. | |||
|
What are the possible side effects of LITFULO? LITFULO may cause serious side effects, including: See**"What is the most important information I should know about LITFULO?"** The most common side effects of LITFULO include: | |||
|
• • • • |
• • • • |
• • • • | |
|
These are not all the possible side effects of LITFULO. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. You may also report side effects to Pfizer at 1-800-438-1985. | |||
|
How should I store LITFULO? • • • Keep LITFULO and all medicines out of the reach of children. | |||
|
General information about the safe and effective use of LITFULO. Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use LITFULO for a condition for which it was not prescribed. Do not give LITFULO to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about LITFULO that is written for health professionals. | |||
|
What are the ingredients in LITFULO?
LAB-1525-1.0 |
DESCRIPTION SECTION
11 DESCRIPTION
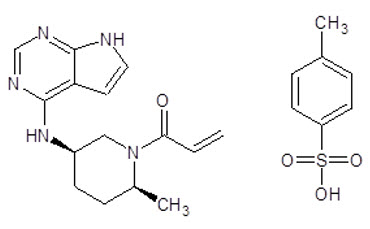
LITFULO (ritlecitinib) capsules are formulated with ritlecitinib tosylate, a kinase inhibitor.
Ritlecitinib tosylate is a white to off white to pale pink solid which is freely soluble in water. The chemical name is 1-{(2S,5R)-2-Methyl-5-[(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino]piperidin-1-yl}prop-2-en-1-one 4 methylbenzene-1-sulfonic acid.
The molecular formula for ritlecitinib tosylate is C22H27N5O4S. The molecular weight is 457.55 g/mol and its structural formula is:
LITFULO is supplied for oral administration as a 50 mg immediate-release capsule. Each capsule contains 50 mg ritlecitinib (equivalent to 80.13 mg ritlecitinib tosylate) and the following inactive ingredients: crospovidone, glyceryl dibehenate, lactose monohydrate, microcrystalline cellulose, and hypromellose (HPMC) capsule shells. The yellow/blue, opaque capsule shells contain Brilliant blue FCF – FD&C Blue, hypromellose, titanium dioxide, and yellow iron oxide.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
The efficacy and safety of LITFULO were evaluated in one randomized, double- blind, placebo-controlled trial (Trial AA-I) in subjects 12 years of age and older with alopecia areata with ≥50% scalp hair loss, including alopecia totalis (AT) and alopecia universalis (AU).
Trial AA-I evaluated a total of 718 subjects who were randomized to one of the following treatment regimens for 48 weeks: 1) 200 mg once daily for 4 weeks followed by 50 mg once daily for 44 weeks; 2) 200 mg once daily for 4 weeks followed by 30 mg once daily for 44 weeks; 3) 50 mg once daily for 48 weeks; 4) 30 mg once daily for 48 weeks; 5) 10 mg once daily for 48 weeks; 6) placebo for 24 weeks followed by 200 mg once daily for 4 weeks and 50 mg once daily for 20 weeks; or 7) placebo for 24 weeks followed by 50 mg once daily for 24 weeks.
The recommended dose of LITFULO is 50 mg once daily and the results for this dose are discussed below.
Across all treatment groups 62% of subjects were female, 68% were White, 26% were Asian, and 4% were Black or African American. The majority of subjects (85%) were adults (≥18 years of age) with a mean age of 33.7 years. A total of 105 (15%) subjects 12 to <18 years of age and 20 (3%) subjects 65 years of age and older were enrolled. The mean baseline Severity of Alopecia Tool (SALT) score ranged from 88.3 to 93.0 across treatment groups; among subjects without AT/AU at baseline, the mean SALT score ranged from 78.3 to 87.0. The majority of subjects had abnormal eyebrows (83%) and eyelashes (75%) at baseline across treatment groups. The median duration since alopecia areata diagnosis was 6.9 years and the median duration of the current alopecia areata episode was 2.5 years. Randomization was stratified by AT/AU status with 46% of subjects classified as AT/AU based upon a baseline SALT score of 100.
Clinical Response
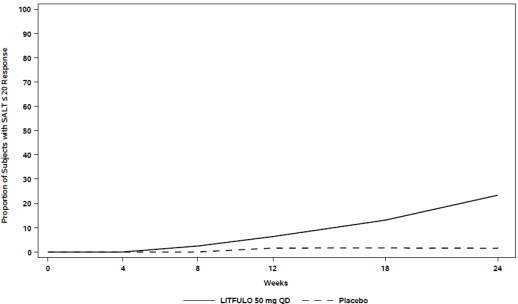
Assessment of scalp hair loss was based on the SALT score. At Week 24, a greater proportion of subjects had a SALT ≤20 response (20% or less of scalp hair loss) and SALT ≤10 response (10% or less of scalp hair loss) with LITFULO compared to placebo (Table 7). The percentage of subjects achieving SALT ≤20 response by visit is shown in Figure 1.
Table 7. Proportion of Subjects with Response on the SALT Scale at Week 24|
LITFULO 50 mg QD |
Placebo |
Difference from Placebo | |
|---|---|---|---|
|
Abbreviations: CI = confidence interval; N = total number of subjects; QD = once daily; SALT = Severity of Alopecia Tool. | |||
| |||
|
SALT ≤20 response* |
23.0 |
1.6 |
21.4 (13.4, 29.5) |
|
SALT ≤10 response† |
13.4 |
1.5 |
11.9 (5.4, 18.3) |
Figure 1. SALT ≤20 Response through Week 24
|
Abbreviations: QD = once daily; SALT = Severity of Alopecia Tool. |
|
|