Albuterol Sulfate
Albuterol SulfateInhalation Solution,0.5%*Sterile(*Potency expressed as albuterol)
953ab65b-b157-41b3-8f90-98fa9d7f20c5
HUMAN PRESCRIPTION DRUG LABEL
Sep 2, 2010
Rebel Distributors Corp.
DUNS: 118802834
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Albuterol Sulfate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
Drug Labeling Information
INDICATIONS & USAGE SECTION
INDICATIONS AND USAGE
Albuterol sulfate inhalation solution is indicated for the relief of bronchospasm in patients 2 years of age and older with reversible obstructive airway disease and acute attacks of bronchospasm.
CONTRAINDICATIONS SECTION
CONTRAINDICATIONS
Albuterol sulfate inhalation solution is contraindicated in patients with a history of hypersensitivity to albuterol or any of its components.
WARNINGS SECTION
WARNINGS
Paradoxical Bronchospasm
Albuterol sulfate inhalation solution can produce paradoxical bronchospasm, which may be life threatening. If paradoxical bronchospasm occurs, albuterol sulfate inhalation solution should be discontinued immediately and alternative therapy instituted. It should be recognized that paradoxical bronchospasm, when associated with inhaled formulations, frequently occurs with the first use of a new canister or vial.
Fatalities have been reported in association with excessive use of inhaled sympathomimetic drugs and with the home use of nebulizers. It is therefore essential that the physician instruct the patient in the need for further evaluation if his/her asthma becomes worse.
Cardiovascular Effects
Albuterol sulfate inhalation solution, like all other beta-adrenergic agonists, can produce a clinically significant cardiovascular effect in some patients as measured by pulse rate, blood pressure, and/or symptoms. Although such effects are uncommon after administration of albuterol sulfate inhalation solution at recommended doses, if they occur, the drug may need to be discontinued. In addition, beta-agonists have been reported to produce electrocardiogram (ECG) changes, such as flattening of the T wave, prolongation of the QTC interval, and ST segment depression. The clinical significance of these findings is unknown. Therefore, albuterol sulfate inhalation solution, like all sympathomimetic amines, should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension.
Deterioration of Asthma
Asthma may deteriorate acutely over a period of hours or chronically over several days or longer. If the patient needs more doses of albuterol sulfate inhalation solution than usual, this may be a marker of destabilization of asthma and requires reevaluation of the patient and treatment regimen, giving special consideration to the possible need for anti-inflammatory treatment, e.g., corticosteroids.
Immediate Hypersensitivity Reactions
Immediate hypersensitivity reactions may occur after administration of albuterol, as demonstrated by rare cases of urticaria, angioedema, rash, bronchospasm, and oropharyngeal edema.
Use of Anti-inflammatory Agents
The use of beta-adrenergic agonist bronchodilators alone may not be adequate to control asthma in many patients. Early consideration should be given to adding anti-inflammatory agents, eg, corticosteroids.
Microbial Contamination
It is recommended that each multi-dose bottle of albuterol be used for only one patient. Nosocomial outbreaks of pneumonia have occurred in hospitals when one multi-dose bottle of albuterol was used to treat more than one patient. To avoid microbial contamination, proper aseptic technique should be used each time the bottle is opened. Precautions should be taken to prevent contact of the dropper tip of the bottle with any surface, including the nebulizer reservoir and associated ventilatory equipment. In addition, if the solution changes color or becomes cloudy, it should not be used.
SPL UNCLASSIFIED SECTION
Patient Instructions For Use
Albuterol Sulfate Inhalation Solution, 0.5%*
*Potency expressed as albuterol
Note: The Albuterol Sulfate Inhalation Solution contained in the 20 mL multiple-dose bottles is concentrated and must be diluted.
Read complete instructions carefully before****using.
**1.**Draw the appropriate volume of Albuterol SulfateInhalation Solution, 0.5% into the specially marked dropper that comes with each multidose bottle (Figure 1). For children 12 years of age and under, the volume is based upon body weight. Use the dropper volume prescribed by your doctor.
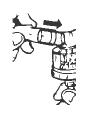
Figure 1
2**.**Squeeze the solution into the nebulizer reservoir through the appropriate opening, taking care not to touch the tip of the dropper (Figure 2).
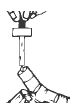
Figure 2
3**.**Add sterile normal saline solution, as your doctor has directed. A general guideline for the amount of saline to add is: For children using 0.25 mL or 1.25 mg of albuterol sulfate inhalation solution, add 2.75 mL of sterile normal saline. For children or adults using 0.5mL or 2.5mg of albuterol sulfate inhalation solution, add 2.5 mL of sterile normal saline.
**4.**Gently swirl the nebulizer to mix the contents and connect it with the mouthpiece or face mask (Figure 3).
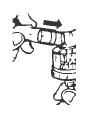
Figure 3
5**.**Connect the nebulizer to the compressor.
**6.**Sit in a comfortable, upright position; place the mouthpiece in your mouth (Figure 4) (or put on the face mask); and turn on the compressor.
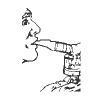
Figure 4
7. Breathe as calmly, deeply, and evenlyas possible until no more mist is formed in the nebulizer chamber (about 5 to 15 minutes). At this point, the treatment is finished.
**8.**Clean the nebulizer (see manufacturer’s instructions).
Note: Use only as directed by your physician.More frequent administration or higherdoses are not recommended.
Store Albuterol Sulfate Inhalation Solution, 0.5% between 2° and 25°C (36°- 77° F).
To avoid microbial contamination, proper aseptic techniques should be used each time the bottle is opened. Precautions should be taken to prevent contact of the dropper tip of the bottle with any surface, including the nebulizer reservoir and associated ventilatory equipment. In addition, if the solution changes color or becomes cloudy, it should not be used. The safety and effectiveness of albuterol sulfate inhalation solution have not been determined when one or more drugs are mixed with it in a nebulizer. Check with your doctor beforemixing anymedications in your nebulizer.
Call your doctor for medical advice about side effects. You may report side effects to Hi-Tech Pharmacal Co., Inc. at 1-800-262-9010 or FDA at 1-800-FDA-1088.
ADDITIONAL INSTRUCTIONS:
Hi-Tech Pharmacal Co., Inc.
Amityville, NY 11701
Rev. 741:03 9/09
MG #11208
Repackaged by:
Rebel Distributors Corp.
Thousand Oaks, CA 91320
