ISENTRESS
These highlights do not include all the information needed to use ISENTRESS safely and effectively. See full prescribing information for ISENTRESS. ISENTRESS (raltegravir) film-coated tablets, for oral use ISENTRESS (raltegravir) chewable tablets, for oral use ISENTRESS (raltegravir) for oral suspension Initial U.S. Approval: 2007
46ef8e2e-ae63-6a3c-e054-00144ff8d46c
HUMAN PRESCRIPTION DRUG LABEL
Jul 17, 2023
NuCare Pharmaceuticals,Inc.
DUNS: 010632300
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
RALTEGRAVIR
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (15)
Drug Labeling Information
DESCRIPTION SECTION
11 DESCRIPTION
ISENTRESS contains raltegravir potassium, a human immunodeficiency virus integrase strand transfer inhibitor. The chemical name for raltegravir potassium is N-[(4-Fluorophenyl) methyl]-1,6-dihydro-5-hydroxy-1-methyl-2-[1-methyl-1-[[(5-methyl-1,3,4-oxadiazol-2-yl)carbonyl]amino]ethyl]-6-oxo-4-pyrimidinecarboxamide monopotassium salt.
The empirical formula is C 20H 20FKN 6O 5 and the molecular weight is 482.51. The structural formula is:
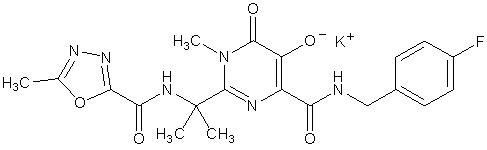
Raltegravir potassium is a white to off-white powder. It is soluble in water, slightly soluble in methanol, very slightly soluble in ethanol and acetonitrile and insoluble in isopropanol.
Each 400 mg film-coated tablet of ISENTRESS for oral administration contains 434.4 mg of raltegravir (as potassium salt), equivalent to 400 mg of raltegravir free phenol and the following inactive ingredients: calcium phosphate dibasic anhydrous, hypromellose 2208, lactose monohydrate, magnesium stearate, microcrystalline cellulose, poloxamer 407 (contains 0.01% butylated hydroxytoluene as antioxidant), sodium stearyl fumarate. In addition, the film coating contains the following inactive ingredients: black iron oxide, polyethylene glycol 3350, polyvinyl alcohol, red iron oxide, talc and titanium dioxide.
Each 100 mg chewable tablet of ISENTRESS for oral administration contains 108.6 mg of raltegravir (as potassium salt), equivalent to 100 mg of raltegravir free phenol and the following inactive ingredients: ammonium hydroxide, crospovidone, ethylcellulose 20 cP, fructose, hydroxypropyl cellulose, hypromellose 2910/6cP, magnesium stearate, mannitol, medium chain triglycerides, monoammonium glycyrrhizinate, natural and artificial flavors (orange, banana, and masking that contains aspartame), oleic acid, PEG 400, red iron oxide, saccharin sodium, sodium citrate dihydrate, sodium stearyl fumarate, sorbitol, sucralose and yellow iron oxide.
Each 25 mg chewable tablet of ISENTRESS for oral administration contains 27.16 mg of raltegravir (as potassium salt), equivalent to 25 mg of raltegravir free phenol and the following inactive ingredients: ammonium hydroxide, crospovidone, ethylcellulose 20 cP, fructose, hydroxypropyl cellulose, hypromellose 2910/6cP, magnesium stearate, mannitol, medium chain triglycerides, monoammonium glycyrrhizinate, natural and artificial flavors (orange, banana, and masking that contains aspartame), oleic acid, PEG 400, saccharin sodium, sodium citrate dihydrate, sodium stearyl fumarate, sorbitol, sucralose and yellow iron oxide.
Each packet of ISENTRESS for oral suspension 100 mg, contains 108.6 mg of raltegravir (as potassium salt), equivalent to 100 mg of raltegravir free phenol and the following inactive ingredients: ammonium hydroxide, banana with other natural flavors, carboxymethylcellulose sodium, crospovidone, ethylcellulose 20 cP, fructose, hydroxypropyl cellulose, hypromellose 2910/6cP, macrogol/PEG 400, magnesium stearate, maltodextrin, mannitol, medium chain triglycerides, microcrystalline cellulose, monoammonium glycyrrhizinate, oleic acid, sorbitol, sucralose and sucrose.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
ISENTRESS tablets 400 mg are pink, oval-shaped, film-coated tablets with "227" on one side. They are supplied as follows:
*NDC 68071-2113-6 bottles of 6
Storage and Handling
400 mg Film-coated Tablets, Chewable Tablets and For Oral Suspension
Store at 20-25°C (68-77°F); excursions permitted to 15-30°C (59-86°F). See USP Controlled Room Temperature.
Chewable Tablets
Store in the original package with the bottle tightly closed. Keep the desiccant in the bottle to protect from moisture.
For Oral Suspension
Store in the original container. Do not open foil packet until ready for use.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise patients to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
General Information
Instruct patients to reread patient labeling each time the prescription is renewed.
Patients should remain under the care of a physician when using ISENTRESS. Instruct patients to inform their physician or pharmacist if they develop any unusual symptom, or if any known symptom persists or worsens.
ISENTRESS is not a cure for HIV-1 infection and patients may continue to experience illnesses associated with HIV-1 infection such as opportunistic infections. Tell patients that sustained decreases in plasma HIV RNA have been associated with a reduced risk of progression to AIDS and death. Patients should remain on continuous HIV therapy to control HIV infection and decrease HIV-related illnesses.
Advise patients to avoid doing things that can spread HIV-1 infection to others.
- Do not share needles or other injection equipment.
- Do not share personal items that can have blood or body fluids on them, like toothbrushes and razor blades.
- Do not have any kind of sex without protection. Always practice safe sex by using a latex or polyurethane condom to lower the chance of sexual contact with semen, vaginal secretions, or blood.
- Do not breastfeed. Mothers with HIV-1 should not breastfeed because HIV-1 can be passed to the baby in the breast milk. Also, it is unknown if ISENTRESS can be passed to the baby through breast milk and whether it could harm the baby.
General Dosing Instructions
Instruct patients that if they miss a dose of ISENTRESS, they should take it as soon as they remember. If they do not remember until it is time for the next dose, instruct them to skip the missed dose and go back to the regular schedule. Instruct patients not to double their next dose or take more than the prescribed dose.
Film-Coated Tablets and Chewable Tablets
Inform patients that the chewable tablet forms can be chewed or swallowed whole, but the film-coated tablets must be swallowed whole.
For Oral Suspension
Instruct parents and/or caregivers to read the Instructions for Use before preparing and administering ISENTRESS for oral suspension to pediatric patients. Instruct parents and/or caregivers that ISENTRESS for oral suspension should be administered within 30 minutes of mixing.
Severe and Potentially Life-threatening Rash
Inform patients that severe and potentially life-threatening rash has been reported. Advise patients to immediately contact their healthcare provider if they develop rash. Instruct patients to immediately stop taking ISENTRESS and other suspect agents, and seek medical attention if they develop a rash associated with any of the following symptoms as it may be a sign of a more serious reaction such as Stevens-Johnson syndrome, toxic epidermal necrolysis or severe hypersensitivity: fever, generally ill feeling, extreme tiredness, muscle or joint aches, blisters, oral lesions, eye inflammation, facial swelling, swelling of the eyes, lips, mouth, breathing difficulty, and/or signs and symptoms of liver problems (e.g., yellowing of the skin or whites of the eyes, dark or tea colored urine, pale colored stools/bowel movements, nausea, vomiting, loss of appetite, or pain, aching or sensitivity on the right side below the ribs). Inform patients that if severe rash occurs, their physician will closely monitor them, order laboratory tests and initiate appropriate therapy.
Rhabdomyolysis
Before patients begin ISENTRESS, ask them if they have a history of rhabdomyolysis, myopathy or increased creatine kinase or if they are taking medications known to cause these conditions such as statins, fenofibrate, gemfibrozil or zidovudine.
Instruct patients to immediately report to their healthcare provider any unexplained muscle pain, tenderness, or weakness while taking ISENTRESS.
Phenylketonuria
Alert patients with phenylketonuria that ISENTRESS Chewable Tablets contain phenylalanine [see Warnings and Precautions (5.3)].
Drug Interactions
Instruct patients to avoid taking aluminum and/or magnesium containing antacids during treatment with ISENTRESS [see Drug Interactions (7.2)] .
INSTRUCTIONS FOR USE SECTION
Instructions for Use
ISENTRESS**®** (eyesen tris)
(raltegravir)
for oral suspension
Read this Instructions for Use before you mix and give a dose of ISENTRESS for oral suspension to your child for the first time, and each time you get a refill. There may be new information. These instructions will help you to correctly mix and give a dose of ISENTRESS for oral suspension to your child.
See the Patient Information leaflet that comes with ISENTRESS for oral suspension for more information about ISENTRESS.
Your doctor will decide the right dose based on your child's weight.
Ask your doctor or pharmacist if you have any questions about how to mix or give ISENTRESS for oral suspension to your child.
|
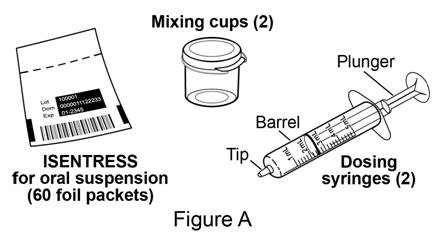
Each ISENTRESS for oral suspension kit contains the following supplies (see **** Figure A**):**
|
|
|
|
For each dose of ISENTRESS for oral suspension you will need the following: |
|
|
How do I prepare a dose of ISENTRESS for oral suspension? |
|
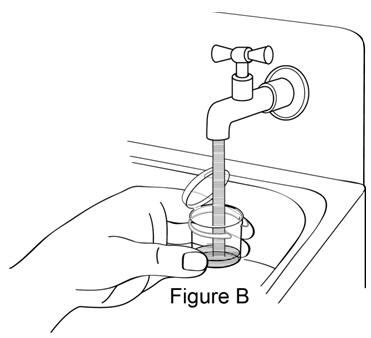
**Step 1.**Fill mixing cup about half-way with drinking water (see Figure B). |
|
|
|
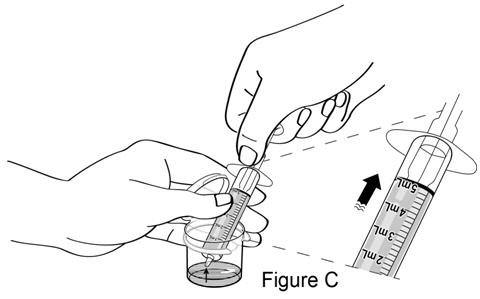
**Step 2.**Fill the dosing syringe. Start with the plunger pushed all the way inside the barrel of the syringe. Insert the tip of the syringe into the water and pull back on the plunger to the 5 mL marking on the barrel of the syringe (see Figure C). |
|
|
|
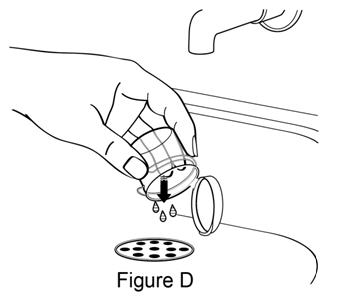
**Step 3.**Pour out remaining water from mixing cup (see Figure D). |
|
|
|
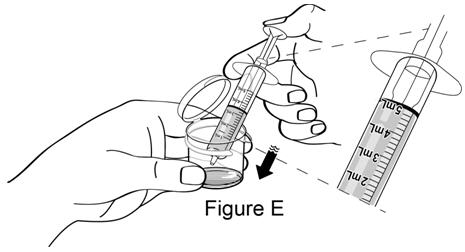
**Step 4.**Add the 5 mL of water from the dosing syringe back into the mixing cup by pressing down on the plunger (see Figure E). |
|
|
|
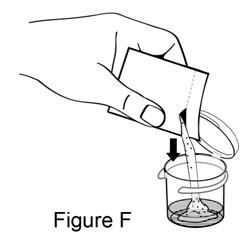
**Step 5.**Open 1 foil packet. There is a notch that you can use to tear open the foil packet, or you may use scissors to cut along the dotted line. Pour entire contents into mixing cup (see Figure F). |
|
|
|
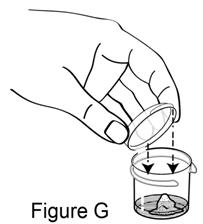
**Step 6.**Close the attached lid to seal the mixing cup (see Figure G). It will snap shut. |
|
|
|
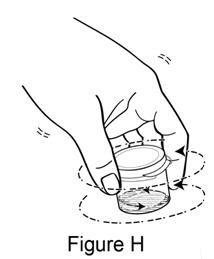
**Step 7.**Swirl the mixing cup to mix using a gentle circular motion for 30-60 seconds (see Figure H).Do not turn the mixing cup upside down. The liquid will be cloudy. |
|
|
|
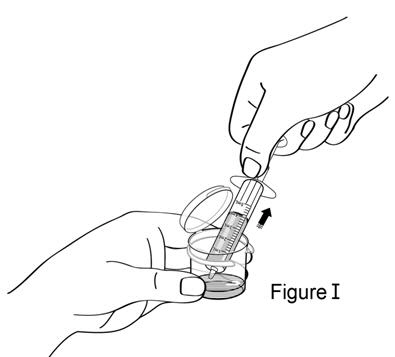
Step 8.Open the mixing cup. Put the tip of the syringe into the liquid andpull back the plunger to the mL marking that matches your child's prescribed dose (see Figure I). Your child's dose may be different from the one shown in the figure. |
|
|
|
How should I give a dose of ISENTRESS for oral suspension? |
|
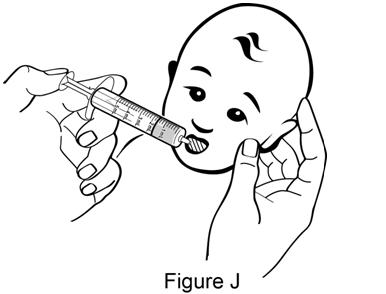
Step 9. Place the tip of the dosing syringe in your child's mouth and turn it toward either cheek. Gently push down on the plunger to give the medicine (see Figure J). Give the dose of ISENTRESS oral suspension to your child within 30 minutes of mixing. If you are not able to give your child's dose within 30 minutes of mixing, pour the unused medicine into the trash. You will need to mix a new dose. |
|
|
|
How should I dispose of leftover ISENTRESS for oral suspension? |
|
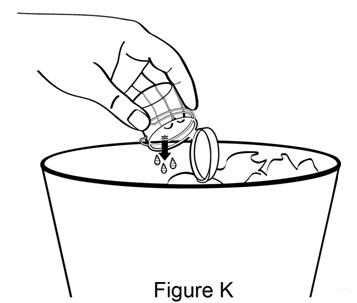
Step 10. Pour any leftover medicine from the mixing cup into the trash (see Figure K). |
|
|
|
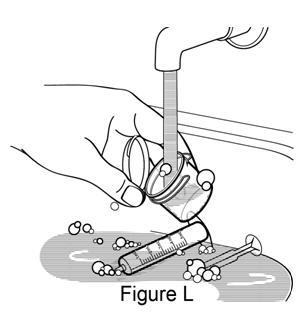
**Step 11.**Remove plunger from the barrel of the dosing syringe. Hand wash the dosing syringe and mixing cup with warm water and dish soap. Rinse with water and air dry (see Figure L). |
|
|
How should I store ISENTRESS for oral suspension?
- Store ISENTRESS for oral suspension at room temperature between 68°F to 77°F (20°C to 25°C).
- Store in the original container. Do not open the foil packets until ready for use.
Keep ISENTRESS for oral suspension and all medicines out of the reach of children.
For more information go to www.ISENTRESS.com or call 1-800-622-4477.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.