Digoxin
Digoxin Oral Solution These highlights do not include all the information needed to use Digoxin Oral Solution safely and effectively. See full prescribing information for Digoxin Oral Solution. Initial U.S. Approval: 1982
dcf907d9-0f0e-4ecc-a555-7e6dcc08c166
HUMAN PRESCRIPTION DRUG LABEL
Nov 21, 2011
Atlantic Biologicals Corps
DUNS: 047437707
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Digoxin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
DIGOXIN SOLUTION

DESCRIPTION SECTION
11 DESCRIPTION
Digoxin is one of the cardiac glycosides, a closely-related group of plant- derived drugs with shared pharmacological effects. The term "digitalis" is used to designate the whole group. Digoxin is extracted from the leaves of the common foxglove, . Like each of the other cardiac glycosides, digoxin consists of a polycyclic core and a sugar side chain. Digoxin’s chemical name is 3β-[ -2,6-dideoxy-β-D- -hexopyranosyl-(1→4)- -2, 6-dideoxy-β-D- -hexopyranosyl-(1→4)-2, 6-dideoxy-β-D- -hexopyranosyl)oxy]-12β, 14-dihydroxy-5β-card-20(22)-enolide; its structural formula is: Digitalis lanata0ribo0riboribo
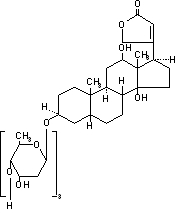
Its molecular formula is C H O , and its molecular weight is 780.95. Digoxin is practically insoluble in water and in ether, slightly soluble in 50% ethanol and in chloroform, and freely soluble in pyridine. Digoxin powder consists of odorless white crystals. 416414
Digoxin Oral Solution, USP is formulated for oral administration, and each mL contains 50 mcg (0.05 mg digoxin). The lime-flavored solution contains the following inactive ingredients: alcohol 10% (by volume at 60°F), glycerin, lime (imitation), methylparaben 0.1%, propylparaben 0.02%, purified water, sodium citrate, and sorbitol solution.
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Considerations
The dose of digoxin should be based on clinical assessment but individual patient factors should be taken into consideration. Those factors are:
- Lean body weight
- Renal function
- Patient age
- Concurrent disease ] [see Warnings and Precautions (5) * Concomitant medication [see ] Drug Interactions (7)
Because the pharmacokinetics of digoxin are complex, and because toxic levels of digoxin are only slightly higher than therapeutic levels, digoxin dosing can be difficult. The recommended approach is to
- estimate the patient’s daily maintenance dose
- adjust the estimate to account for patient-specific factors
- choose a dosing regimen
- decide whether to initiate therapy with a loading dose
- monitor the patient for toxicity and for therapeutic effect
- adjust the dose
Dose titration may be accomplished by either of two general approaches that differ in dosage and frequency of administration, but reach the same total amount of digoxin accumulated in the body.
- If rapid titration is considered medically appropriate, administer a loading dose based upon projected peak digoxin body stores. Maintenance dose can be calculated as a percentage of the loading dose.
- More gradual titration may be obtained by beginning an appropriate maintenance dose, thus allowing digoxin body stores to accumulate slowly. Steady-state serum digoxin concentrations will be achieved in approximately five half-lives of the drug for the individual patient. Depending upon the patient’s renal function, this will take between 1 and 3 weeks.
2.2 Serum Digoxin Concentrations
In general, the dose of digoxin used should be determined on clinical grounds. However, measurement of serum digoxin concentrations can be helpful to the clinician in determining the adequacy of digoxin therapy and in assigning certain probabilities to the likelihood of digoxin intoxication.
Studies have shown diminished efficacy at serum levels < 0.5 ng/mL, while levels above 2 ng/mL are associated with increased toxicity without increased benefit. The inotropic effects of digoxin tend to appear at lower concentrations than the electrophysiological effects. Based on retrospective analysis, adverse events may be higher in the upper therapeutic range.
Perform sampling of serum concentrations just before the next scheduled dose of the drug. If this is not possible, sample at least 6 hours or later after the last dose, regardless of the route of administration or the formulation used. On a once-daily dosing schedule, the concentration of digoxin will be 10% to 25% lower when sampled at 24 versus 8 hours, depending upon the patient’s renal function. On a twice-daily dosing schedule, there will be only minor differences in serum digoxin concentrations whether sampling is done at 8 or 12 hours after a dose. The serum concentration of digoxin should always be interpreted in the overall clinical context, and an isolated measurement should not be used alone as the basis for increasing or decreasing the dose of the drug.
When decision-making is to be guided by serum digoxin levels, the clinician must consider the possibility of reported concentrations that have been falsely elevated by endogenous digoxin-like immunoreactive substances If the assay being used is sensitive to these substances, it may be prudent to obtain a baseline measurement before digoxin therapy is started, and correct later values by the reported baseline level. [see ] Drug Interactions (7.4).
2.3 Loading Dose
Loading doses for each age group are given in Table 1 below.
In pediatric patients, if a loading dose is needed, it can be administered with roughly half the total given as the first dose. Additional fractions of this planned total dose may be given at 4- to 8-hour intervals, with careful assessment of clinical response before each additional dose. If the patient’s clinical response necessitates a change from the calculated loading dose of digoxin, then calculation of the maintenance dose should be based upon the amount actually given as the loading dose [see and ]. Table 12
Table 1: Estimate the Loading Dose|
Age |
Oral Loading Dose, mcg/kg |
|
Premature |
20 - 30 |
|
Full-Term |
25 - 35 |
|
1 to 24 months |
35 - 60 |
|
2 to 5 years |
30 - 45 |
|
5 to 10 years |
20 - 35 |
|
Over 10 years |
10 - 15 |
More gradual attainment of digoxin levels can also be accomplished by beginning an appropriate maintenance dose. The range of percentages provided in Table 2 (2.4 Estimate of Daily Maintenance Dose) can be used in calculating this dose for patients with normal renal function. Steady state will be attained after approximately 5 days in subjects with normal renal function.
2.4 Estimate of Daily Maintenance Dose
The recommended daily maintenance doses for each age group are given in Table 2 below. These recommendations assume the presence of normal renal function.
Table 2: Estimate of the Daily Maintenance Dose|
Age |
Daily Oral Maintenance Dose, mcg/kg/day |
Dose Regimen, mcg/kg/dose |
|
Premature |
4.7 – 7.8 |
2.3 – 3.9 Twice daily |
|
Full-Term |
7.5 – 11.3 |
3.8 – 5.6 Twice daily |
|
1 to 24 months |
11.3 – 18.8 |
5.6 – 9.4 Twice daily |
|
2 to 5 years |
9.4 – 13.1 |
4.7 – 6.6 Twice daily |
|
5 to 10 years |
5.6 – 11.3 |
2.8 – 5.6 Twice daily |
|
Over 10 years |
3.0 – 4.5 |
3.0 – 4.5 Once daily |
Dosage guidelines provided are based upon average patient response and substantial individual variation can be expected. Accordingly, dosage selection must be based upon clinical assessment and ultimately therapeutic drug level monitoring of the patient.
Divided daily dosing is recommended for pediatric patients under age 10. In the newborn period, renal clearance of digoxin is diminished and suitable dosage adjustments must be made as shown in Tables 1 and 2. Renal clearance is further reduced in the premature infant. Beyond the immediate newborn period, pediatric patients generally require proportionally larger doses than adults on the basis of body weight or body surface area. Pediatric patients over 10 years of age require adult dosages in proportion to their body weight. Some researchers have suggested that infants and young pediatric patients tolerate slightly higher serum concentrations than do adults. For pediatric patients with known or suspected renal dysfunction, lower starting doses should be considered combined with frequent monitoring of digoxin levels.
The calibrated dropper supplied with the 60 mL bottle of digoxin oral solution is not appropriate to measure doses below 0.2 mL. Doses less than 0.2 mL require appropriate methods or measuring devices designed to administer an accurate amount to the patient, such as a graduated syringe.NOTE:
2.5 Adjustment of Dose
The body’s handling of digoxin can be affected by many different patient- specific factors. Some of the possible effects are small, so anticipatory dose adjustment might not be required, but others should be considered before initial dosing . [see and Clinical Pharmacology (12.2)] Drug Interactions (7)
Both adults and pediatric patients with abnormal renal function need to have the dose of digoxin proportionally reduced. Recommended maintenance doses based upon lean body weight and renal function are listed in . Developmental changes in pediatric renal function were factored into Table 3. However, age- related and other changes in adult renal function were not. Table 3
The volume of distribution of digoxin is proportional to lean body weight and doses listed in Table 3 assume average body composition. The dose of digoxin must be reduced in patients whose lean weight is an abnormally small fraction of their total body mass because of obesity or edema.
Table 3: Usual Maintenance Dose Requirements (mcg) of Digoxin Based upon Age, Lean Body Weight and Renal Function
| ||||||||||||||||
|
Corrected Ccr (mL/min per 70 kg)***** |
Dose to be given †Twice Daily**‡** |
Dose to be givenOnce Daily |
Number of Days Before Steady State Achieve | |||||||||||||
|
< 10 years of age |
> 10 years of age and adults | |||||||||||||||
|
Lean Body Weight |
Lean Body Weight | |||||||||||||||
|
kg |
5 |
10 |
20 |
30 |
40 |
50 |
60 |
40 |
50 |
60 |
70 |
80 |
90 |
100 | ||
|
lb |
11 |
22 |
44 |
66 |
88 |
110 |
132 |
88 |
110 |
132 |
154 |
176 |
198 |
220 | ||
|
10 |
10 |
20 |
40 |
60 |
80 |
100 |
120 |
80 |
100 |
120 |
140 |
160 |
180 |
200 |
19 | |
|
20 |
11 |
23 |
45 |
68 |
90 |
113 |
135 |
90 |
113 |
135 |
158 |
180 |
203 |
225 |
16 | |
|
30 |
13 |
25 |
50 |
75 |
100 |
125 |
150 |
100 |
125 |
150 |
175 |
200 |
225 |
250 |
14 | |
|
40 |
14 |
28 |
55 |
83 |
110 |
138 |
165 |
110 |
138 |
165 |
193 |
220 |
248 |
275 |
13 | |
|
50 |
15 |
30 |
60 |
90 |
120 |
150 |
180 |
120 |
150 |
180 |
210 |
240 |
270 |
300 |
12 | |
|
60 |
16 |
33 |
65 |
98 |
130 |
163 |
195 |
130 |
163 |
195 |
228 |
260 |
293 |
325 |
11 | |
|
70 |
18 |
35 |
70 |
105 |
140 |
175 |
210 |
140 |
175 |
210 |
245 |
280 |
315 |
350 |
10 | |
|
80 |
19 |
38 |
75 |
113 |
150 |
188 |
225 |
150 |
188 |
225 |
263 |
300 |
338 |
375 |
9 | |
|
90 |
20 |
40 |
80 |
120 |
160 |
200 |
240 |
160 |
200 |
240 |
280 |
320 |
360 |
400 |
8 | |
|
100 |
21 |
43 |
85 |
128 |
170 |
213 |
255 |
170 |
213 |
255 |
298 |
340 |
383 |
425 |
7 |
Determination of the target dose in milliliters of Digoxin Oral Solution based on body weight is shown in . Provided is the volume required per dose, NOT per day. Table 4
Table 4: Dose in Milliliters|
|
Volume to be given in mL | |||||||||||||
|
2 |
3 |
4 |
5 |
6 |
8 |
10 |
12 |
14 |
16 |
18 |
20 |
30 | ||
|
|
2 |
0.08 b |
0.12 b |
0.16 b |
0.2 |
0.2 |
0.3 |
0.4 |
0.5 |
0.6 |
0.6 |
0.7 |
0.8 |
1.2 |
|
3 |
0.12 b |
0.18 b |
0.2 |
0.3 |
0.4 |
0.5 |
0.6 |
0.7 |
0.8 |
1.0 |
1.1 |
1.2 |
1.8 | |
|
4 |
0.16 b |
0.2 |
0.3 |
0.4 |
0.5 |
0.6 |
0.8 |
1.0 |
1.1 |
1.3 |
1.4 |
1.6 |
2.4 | |
|
5 |
0.2 |
0.3 |
0.4 |
0.5 |
0.6 |
0.8 |
1.0 |
1.2 |
1.4 |
1.6 |
1.8 |
2.0 |
3.0 | |
|
6 |
0.2 |
0.4 |
0.5 |
0.6 |
0.7 |
1.0 |
1.2 |
1.4 |
1.7 |
1.9 |
2.2 |
2.4 |
3.6 | |
|
7 |
0.3 |
0.4 |
0.6 |
0.7 |
0.8 |
1.1 |
1.4 |
1.7 |
2.0 |
2.2 |
2.5 |
2.8 |
4.2 | |
|
8 |
0.3 |
0.5 |
0.6 |
0.8 |
1.0 |
1.3 |
1.6 |
1.9 |
2.2 |
2.6 |
2.9 |
3.2 |
4.8 | |
|
9 |
0.4 |
0.5 |
0.7 |
0.9 |
1.1 |
1.4 |
1.8 |
2.2 |
2.5 |
2.9 |
3.2 |
3.6 |
5.4 | |
|
10 |
0.4 |
0.6 |
0.8 |
1.0 |
1.2 |
1.6 |
2.0 |
2.4 |
2.8 |
3.2 |
3.6 |
4.0 |
6.0 | |
|
11 |
0.4 |
0.7 |
0.9 |
1.1 |
1.3 |
1.8 |
2.2 |
2.6 |
3.1 |
3.5 |
4.0 |
4.4 |
6.6 | |
|
12 |
0.5 |
0.7 |
1.0 |
1.2 |
1.4 |
1.9 |
2.4 |
2.9 |
3.4 |
3.8 |
4.3 |
4.8 |
7.2 | |
|
13 |
0.5 |
0.8 |
1.0 |
1.3 |
1.6 |
2.1 |
2.6 |
3.1 |
3.6 |
4.2 |
4.7 |
5.2 |
7.8 | |
|
14 |
0.6 |
0.8 |
1.1 |
1.4 |
1.7 |
2.2 |
2.8 |
3.4 |
3.9 |
4.5 |
5.0 |
5.6 |
8.4 | |
|
15 |
0.6 |
0.9 |
1.2 |
1.5 |
1.8 |
2.4 |
3.0 |
3.6 |
4.2 |
4.8 |
5.4 |
6.0 |
9.0 | |
|
20 |
0.8 |
1.2 |
1.6 |
2.0 |
2.4 |
3.2 |
4.0 |
4.8 |
5.6 |
6.4 |
7.2 |
8.0 |
12.0 | |
|
30 |
1.2 |
1.8 |
2.4 |
3.0 |
3.6 |
4.8 |
6.0 |
7.2 |
8.4 |
9.6 |
10.8 |
12.0 |
18.0 | |
|
40 |
1.6 |
2.4 |
3.2 |
4.0 |
4.8 |
6.4 |
8.0 |
9.6 |
11.2 |
12.8 |
14.4 |
16.0 |
24.0 | |
|
50 |
2.0 |
3.0 |
4.0 |
5.0 |
6.0 |
8.0 |
10.0 |
12.0 |
14.0 |
16.0 |
18 |
20.0 |
30.0 | |
|
60 |
2.4 |
3.6 |
4.8 |
6.0 |
7.2 |
9.6 |
12.0 |
14.4 |
16.8 |
19.2 |
21.6 |
24.0 |
36.0 | |
|
70 |
2.8 |
4.2 |
5.6 |
7.0 |
8.4 |
11.2 |
14.0 |
16.8 |
19.6 |
22.4 |
25.2 |
28.0 |
42.0 | |
|
80 |
3.2 |
4.8 |
6.4 |
8.0 |
9.6 |
12.8 |
16.0 |
19.2 |
22.4 |
25.6 |
28.8 |
32.0 |
48.0 | |
|
90 |
3.6 |
5.4 |
7.2 |
9.0 |
10.8 |
14.4 |
18.0 |
21.6 |
25.2 |
28.8 |
32.4 |
36.0 |
54.0 | |
|
100 |
4.0 |
6.0 |
8.0 |
10.0 |
12.0 |
16.0 |
20.0 |
24.0 |
28.0 |
32.0 |
36.0 |
40.0 |
60.0 |
Recommended dosing regimen for pediatric patients under 10 years of age is twice daily. Recommended dosing regimen for pediatric patients over 10 years of age and adults is once daily. a
Use calibrated dropper for measurement. In the case of required volume less than 0.2 mL, a separate device such as a graduated syringe is recommended for adequate measurement. b
On the left side of the chart, locate the patient’s weight in kilograms. At the top of the chart, identify which dose in mcg/kg will be used for this patient. The block on the chart at which the two rows (weight and target dose) intersect is the milliliter amount that should be given to the patient.
The monitoring described in Section 2.2 may suggest increases or decreases in digoxin doses. Additional monitoring, and in some cases anticipatory dose adjustment, may be indicated around the time of various changes to the patient including:
- normal development through childhood;
- concomitant drug use should be considered when adjusting the estimated digoxin dose ; [see] Drug Interactions (7)
- new co-administration of an antibiotic, especially if the patient had required high doses of digoxin in order to achieve modest serum concentrations, raising the suspicion that a substantial fraction of administered digoxin was being destroyed by colonic bacteria; and
- changes in renal function . [see above] Table 3: Usual Maintenance Dose Requirements (mcg) of Digoxin
Toxic levels of digoxin are only slightly higher than therapeutic levels. The pharmacokinetics of digoxin are complex and dose determination should take into account patient-specific factors (age, lean body weight, renal function, etc.). (2.4)(2.5) Patients should be monitored for toxicity and therapeutic effect and doses should be adjusted, accordingly. (2.2) (2)

 Target
Dose in mcg/kg
Target
Dose in mcg/kg Weight
in kg
Weight
in kg