Praluent
These highlights do not include all the information needed to use PRALUENT safely and effectively. See full prescribing information for PRALUENT. PRALUENT (alirocumab) injection, for subcutaneous use Initial U.S. Approval: 2015
7bcfbac2-e8ac-4569-8edc-bcde3b1fd172
HUMAN PRESCRIPTION DRUG LABEL
Mar 8, 2024
Regeneron Pharmaceuticals, Inc.
DUNS: 194873139
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
alirocumab
PRODUCT DETAILS
INGREDIENTS (5)
alirocumab
PRODUCT DETAILS
INGREDIENTS (5)
Drug Labeling Information
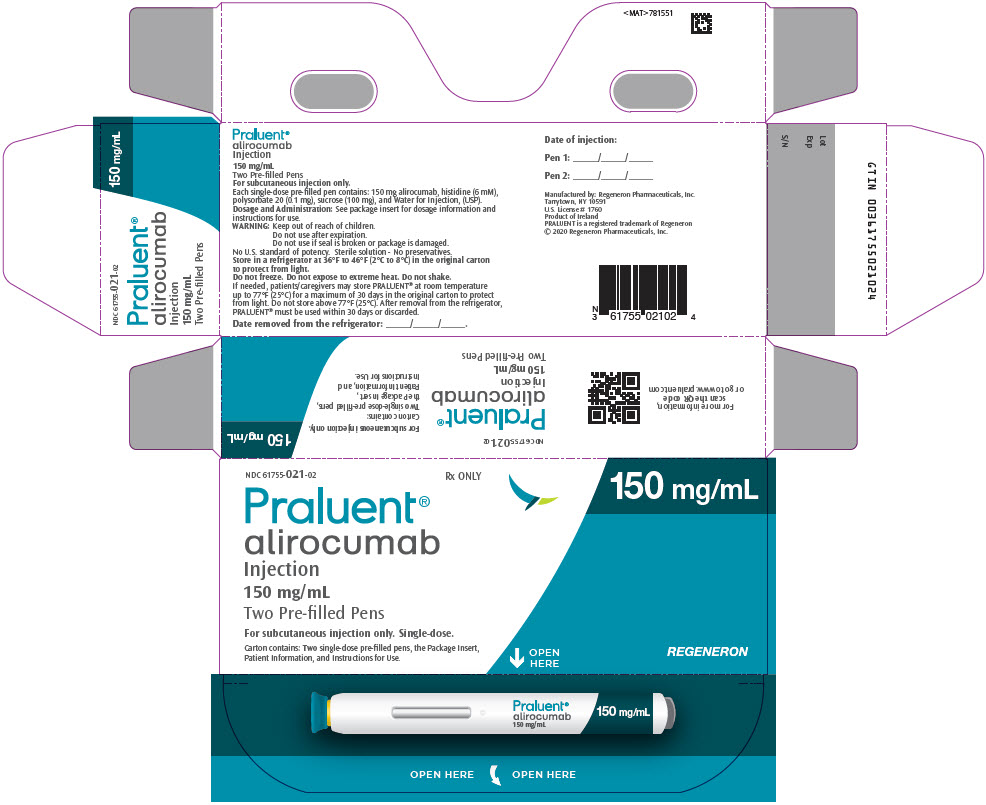
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 150 mg/mL Pen Carton
NDC 61755-021-02
Rx ONLY
150 mg/mL
Praluent®
alirocumab
Injection
150 mg/mL
Two Pre-filled Pens
For subcutaneous injection only. Single-dose.
Carton contains: Two single-dose pre-filled pens, the Package Insert,
Patient Information, and Instructions for Use.
OPEN
HERE
REGENERON
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
PRALUENT® is indicated:
- To reduce the risk of myocardial infarction, stroke, and unstable angina requiring hospitalization in adults with established cardiovascular disease**.**
- As an adjunct to diet, alone or in combination with other low density lipoprotein cholesterol (LDL-C)-lowering therapies, in adults with primary hyperlipidemia, including heterozygous familial hypercholesterolemia (HeFH), to reduce LDL-C.
- As an adjunct to other LDL-C-lowering therapies in adult patients with homozygous familial hypercholesterolemia (HoFH) to reduce LDL-C.
- As an adjunct to diet and other LDL-C-lowering therapies in pediatric patients aged 8 years and older with HeFH to reduce LDL-C.
PRALUENT is a proprotein convertase subtilisin kexin type 9 (PCSK9) inhibitor indicated:
- To reduce the risk of myocardial infarction, stroke, and unstable angina requiring hospitalization in adults with established cardiovascular disease. (1)
- As adjunct to diet, alone or in combination with other low-density lipoprotein cholesterol (LDL-C)-lowering therapies, in adults with primary hyperlipidemia, including heterozygous familial hypercholesterolemia (HeFH), to reduce LDL-C. (1)
- As an adjunct to other LDL-C-lowering therapies in adult patients with homozygous familial hypercholesterolemia (HoFH) to reduce LDL-C. (1)
- As an adjunct to diet and other LDL-C-lowering therapies in pediatric patients aged 8 years and older with HeFH to reduce LDL-C. (1)
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
PRALUENT is contraindicated in patients with a history of a serious hypersensitivity reaction to alirocumab or any of the excipients in PRALUENT. Hypersensitivity vasculitis, angioedema, and hypersensitivity reactions requiring hospitalization have occurred [see Warnings and Precautions (5.1)].
History of a serious hypersensitivity reaction to alirocumab or any of the excipients in PRALUENT. (4)
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Hypersensitivity reactions, including hypersensitivity vasculitis, angioedema, and other hypersensitivity reactions requiring hospitalization, have been reported with PRALUENT treatment. If signs or symptoms of serious hypersensitivity reactions occur, discontinue treatment with PRALUENT, treat according to the standard of care, and monitor until signs and symptoms resolve. PRALUENT is contraindicated in patients with a history of a serious hypersensitivity reaction to alirocumab or any excipient in PRALUENT [see Contraindications (4)].
Hypersensitivity reactions: hypersensitivity vasculitis, angioedema, and other hypersensitivity reactions requiring hospitalization, have been reported with PRALUENT treatment. If signs or symptoms of serious hypersensitivity reactions occur, discontinue treatment with PRALUENT, treat according to the standard of care, and monitor until signs and symptoms resolve. (5.1)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following adverse reactions are also discussed in the other sections of the labeling:
- Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse Reactions in Adults with Primary Hyperlipidemia
The data in Table 1 are derived from 9 primary hyperlipidemia placebo- controlled trials that included 2,476 adult patients treated with PRALUENT 75 mg and/or 150 mg every 2 weeks, including 2,135 exposed for 6 months and 1,999 exposed for more than 1 year (median treatment duration of 65 weeks). The mean age of the population was 59 years, 40% of the population were female, 90% were White, 4% were Black or African American, 3% were Asian, and 3% other races; 6% identified as Hispanic or Latino ethnicity.
Adverse reactions reported in at least 2% of PRALUENT-treated patients, and more frequently than in placebo-treated patients, are shown in Table 1.
Table 1: Adverse Reactions Occurring in >2% of PRALUENT-Treated Adult Patients and ≥1% More Frequently Than with Placebo|
Adverse Reactions |
Placebo |
PRALUENT* |
|---|---|---|
| ||
|
Injection site reactions† |
5 |
7 |
|
Influenza |
5 |
6 |
|
Diarrhea |
4 |
5 |
|
Myalgia |
3 |
4 |
|
Muscle spasms |
2 |
3 |
|
Contusion |
1 |
2 |
Adverse reactions led to discontinuation of treatment in 5.3% of patients treated with PRALUENT and 5.1% of patients treated with placebo. The most common adverse reactions leading to treatment discontinuation in patients treated with PRALUENT were allergic reactions (0.6% versus 0.2% for PRALUENT and placebo, respectively) and elevated liver enzymes (0.3% versus <0.1%).
In an analysis of ezetimibe-controlled trials in which 864 patients were exposed to PRALUENT for a median of 27 weeks and 618 patients were exposed to ezetimibe for a median of 24 weeks, the types and frequencies of common adverse reactions were similar to those listed above.
Adverse Reactions in a Cardiovascular Outcomes Trial in Adults
In a cardiovascular outcomes trial in which 9,451 patients were exposed to PRALUENT for a median of 31 months and 9,443 patients were exposed to placebo for a median of 32 months, common adverse reactions (greater than 5% of patients treated with PRALUENT and occurring more frequently than placebo) included myalgia (6% PRALUENT, 5% placebo).
Adverse Reactions in Pediatric Patients with HeFH
In a 24-week placebo-controlled clinical trial in which 101 pediatric patients aged 8 to 17 years with HeFH were exposed to PRALUENT and 52 pediatric patients with HeFH were exposed to placebo [see Clinical Studies (14.3)], the safety profile of PRALUENT observed in this population was consistent with the safety profile observed in adults with HeFH.
Other Adverse Reactions
Local Injection Site Reactions
In a pool of placebo-controlled trials evaluating PRALUENT 75 mg and/or 150 mg administered every 2 weeks in adults, local injection site reactions including erythema/redness, itching, swelling, and pain/tenderness were reported more frequently in patients treated with PRALUENT (7.2% versus 5.1% for PRALUENT and placebo, respectively). Few patients discontinued treatment because of these reactions (0.2% versus 0.4% for PRALUENT and placebo, respectively), but patients receiving PRALUENT had a greater number of injection site reactions, had more reports of associated symptoms, and had reactions of longer average duration than patients receiving placebo.
In a 48-week placebo-controlled trial evaluating PRALUENT 300 mg every 4 weeks and 75 mg every 2 weeks in adults, in which all patients received an injection of drug or placebo every 2 weeks, local injection site reactions were reported more frequently in patients treated with PRALUENT 300 mg every 4 weeks as compared to those receiving PRALUENT 75 mg every 2 weeks or placebo (16.6%, 9.6%, and 7.9%, respectively). Three patients (0.7%) treated with PRALUENT 300 mg every 4 weeks discontinued treatment due to local injection site reactions versus no patients (0%) in the other 2 treatment groups.
In a cardiovascular outcomes trial in adults, local injection site reactions were reported in 3.8% of patients treated with PRALUENT versus 2.1% patients treated with placebo, and led to permanent discontinuation in 26 patients (0.3%) versus 3 patients (<0.1%), respectively.
In the trial of pediatric patients with HeFH, local injection site reactions were reported in 5% of patients treated with PRALUENT versus 0% patients treated with placebo; no patients discontinued treatment due to injection site reactions.
Hypersensitivity Reactions in Adults
Hypersensitivity reactions were reported more frequently in adult patients treated with PRALUENT than in those treated with placebo (8.6% versus 7.8%). The most common hypersensitivity reaction was pruritus (1.1% versus 0.4% for PRALUENT and placebo, respectively). The proportion of patients who discontinued treatment due to allergic reactions was higher among those treated with PRALUENT (0.6% versus 0.2%).
Serious allergic reactions, such as hypersensitivity, nummular eczema, and hypersensitivity vasculitis were reported in patients using PRALUENT in controlled clinical trials.
Liver Enzyme Abnormalities in Adults
In the primary hyperlipidemia trials in adults, liver-related disorders (primarily related to abnormalities in liver enzymes) were reported in 2.5% of patients treated with PRALUENT and 1.8% of patients treated with placebo, leading to treatment discontinuation in 0.4% and 0.2% of patients, respectively. Increases in serum transaminases to greater than 3 times the upper limit of normal occurred in 1.7% of patients treated with PRALUENT and 1.4% of patients treated with placebo.
6.2 Postmarketing Experience
The following adverse reactions have been reported during post-approval use of PRALUENT. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Hypersensitivity reactions: Angioedema
- Influenza-like illness
Common (>5% of patients treated with PRALUENT and more frequently than placebo) adverse reactions in adults with:
Primary hyperlipidemia: injection site reactions, and influenza. (6) Established cardiovascular disease: myalgia. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Regeneron at 1-844-734-6643 or FDA at 1-800-FDA-1088 or****www.fda.gov/medwatch.
RECENT MAJOR CHANGES SECTION
RECENT MAJOR CHANGES
|
Indications and Usage (1) |
3/2024 |
|
Dosage and Administration (2.2) |
3/2024 |
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
PRALUENT injection is a clear, colorless to pale yellow solution available as follows:
- 75 mg/mL single-dose pre-filled pen
- 150 mg/mL single-dose pre-filled pen
Injection: 75 mg/mL or 150 mg/mL in a single-dose pre-filled pen. (3)
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from clinical trials and postmarketing reports on PRALUENT use in pregnant women are insufficient to evaluate for a drug-associated risk of major birth defects, miscarriage or other adverse maternal or fetal outcomes. In animal reproduction studies, there were no effects on embryo-fetal development when rats were subcutaneously administered alirocumab during organogenesis at dose exposures up to 12-fold the exposure at the maximum recommended human dose of 150 mg every two weeks. In monkeys, suppression of the humoral immune response was observed in infant monkeys when alirocumab was dosed during organogenesis to parturition at dose exposures 13-fold the exposure at the maximum recommended human dose of 150 mg every two weeks. No additional effects on pregnancy or neonatal/infant development were observed at dose exposures up to 81-fold the maximum recommended human dose of 150 mg every two weeks. Measurable alirocumab serum concentrations were observed in the infant monkeys at birth at comparable levels to maternal serum, indicating that alirocumab, like other IgG antibodies, crosses the placental barrier. Monoclonal antibodies are transported across the placenta in increasing amounts especially near term; therefore, alirocumab has the potential to be transmitted from the mother to the developing fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population(s) is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2%-4% and 15%-20%, respectively.
There is a pregnancy safety study for PRALUENT. If PRALUENT is administered during pregnancy, healthcare providers should report PRALUENT exposure by contacting Regeneron at 1-844-734-6643.
Data
Animal data
In Sprague Dawley rats, no effects on embryo-fetal development were observed when alirocumab was dosed at up to 75 mg/kg/dose by the subcutaneous route on gestation days 6 and 12 at exposures 12-fold the maximum recommended human dose of 150 mg every two weeks, based on serum AUC.
In cynomolgus monkeys, suppression of the humoral immune response to keyhole limpet hemocyanin (KLH) antigen was observed in infant monkeys at 4 to 6 months of age when alirocumab was dosed during organogenesis to parturition at 15 mg/kg/week and 75 mg/kg/week by the subcutaneous route, corresponding to 13-fold and 81-fold the human exposure at the maximum recommended human dose of 150 mg every two weeks, based on serum AUC. The lowest dose tested in the monkey resulted in humoral immune suppression; therefore, it is unknown if this effect would be observed at clinical exposure. No study designed to challenge the immune system of infant monkeys was conducted. No additional embryo-fetal, prenatal or postnatal effects were observed in infant monkeys, and no maternal effects were observed, when alirocumab was dosed at up to 75 mg/kg/week by the subcutaneous route, corresponding to maternal exposure of 81-fold the exposure at the maximum recommended human dose of 150 mg every two weeks, based on serum AUC.
8.2 Lactation
Risk Summary
There is no information regarding the presence of alirocumab in human milk, the effects on the breastfed infant, or the effects on milk production. The development and health benefits of breastfeeding should be considered along with the mother's clinical need for PRALUENT and any potential adverse effects on the breastfed infant from PRALUENT or from the underlying maternal condition. Human IgG is present in human milk, but published data suggest that breast milk IgG antibodies do not enter the neonatal and infant circulation in substantial amounts.
8.4 Pediatric Use
The safety and effectiveness of PRALUENT as an adjunct to diet and other LDL- C-lowering therapies for the treatment of HeFH have been established in pediatric patients aged 8 years and older. Use of PRALUENT for this indication is based on data from a 24-week, randomized, placebo-controlled, double-blind trial in pediatric patients with HeFH. In the trial, 101 patients received PRALUENT and 52 patients received placebo; 26 patients (17%) were 8 to 9 years of age. This indication is supported by evidence from controlled trials in adults [see Adverse Reactions (6.1) and Clinical Studies (14.3)].
The safety and effectiveness of PRALUENT have not been established in pediatric patients with HeFH who are younger than 8 years of age or in pediatric patients with other types of hyperlipidemia.
8.5 Geriatric Use
In controlled trials, 3663 patients treated with PRALUENT were ≥65 years of age and 734 patients treated with PRALUENT were ≥75 years of age. No overall differences in safety or effectiveness were observed between these patients and younger patients, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Renal Impairment
No dose adjustment is needed for patients with mild or moderately impaired renal function. No data are available in patients with severe renal impairment [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
No dose adjustment is needed for patients with mild or moderate hepatic impairment. No data are available in patients with severe hepatic impairment [see Clinical Pharmacology (12.3)].
DESCRIPTION SECTION
11 DESCRIPTION
Alirocumab is a human monoclonal antibody (IgG1 isotype) that targets proprotein convertase subtilisin kexin type 9 (PCSK9). Alirocumab is a PCSK9 inhibitor produced by recombinant DNA technology in Chinese Hamster Ovary cell suspension culture. Alirocumab consists of two disulfide-linked human heavy chains, each covalently linked through a disulfide bond to a human kappa light chain. A single N-linked glycosylation site is located in each heavy chain within the CH2 domain of the Fc constant region of the molecule. The variable domains of the heavy and light chains combine to form the PCSK9 binding site within the antibody. Alirocumab has an approximate molecular weight of 146 kDa.
PRALUENT is a sterile, preservative-free, clear, colorless to pale yellow solution for subcutaneous use. PRALUENT 75 mg/mL or 150 mg/mL solution for subcutaneous injection in a single-dose pre-filled pen is supplied in a siliconized 1 mL Type-1 clear glass syringe.
Each 75 mg/mL pre-filled pen contains 75 mg alirocumab, histidine (8 mM), polysorbate 20 (0.1 mg), sucrose (100 mg), and Water for Injection USP, to pH 6.0.
Each 150 mg/mL pre-filled pen contains 150 mg alirocumab, histidine (6 mM), polysorbate 20 (0.1 mg), sucrose (100 mg), and Water for Injection USP, to pH 6.0.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Alirocumab is a human monoclonal antibody that binds to proprotein convertase subtilisin kexin type 9 (PCSK9). PCSK9 binds to the low-density lipoprotein (LDL) receptors (LDLR) on the surface of hepatocytes to promote LDLR degradation within the liver. By inhibiting the binding of PCSK9 to LDLR, alirocumab increases the number of LDLRs available to clear LDL, thereby lowering LDL-C levels.
12.2 Pharmacodynamics
Alirocumab reduced free PCSK9 in a concentration-dependent manner. Following a single subcutaneous administration of alirocumab 75 or 150 mg, maximal suppression of free PCSK9 occurred within 4 to 8 hours. Free PCSK9 concentrations returned to baseline when alirocumab concentrations decreased below the limit of quantitation.
12.3 Pharmacokinetics
Absorption
After subcutaneous administration of 75 mg to 300 mg alirocumab, median times to maximum serum concentrations (tmax) were 3-7 days. The pharmacokinetics of alirocumab after single subcutaneous administration of 75 mg into the abdomen, upper arm, or thigh were similar. The absolute bioavailability of alirocumab after subcutaneous administration was about 85% as determined by population pharmacokinetics analysis. A slightly greater than dose proportional increase was observed, with a 2.1-fold to 2.7-fold increase in total alirocumab concentrations for a 2-fold increase in dose from 75 mg every 2 weeks to 150 mg every 2 weeks. Monthly dose normalized exposure with 300 mg every 4 weeks treatment was similar to that of 150 mg every 2 weeks. Steady state was reached after 2 to 3 doses with an accumulation ratio up to a maximum of about 2-fold.
Distribution
Following intravenous administration, the volume of distribution was about 0.04 to 0.05 L/kg indicating that alirocumab is distributed primarily in the circulatory system.
Elimination
Specific metabolism studies were not conducted, because alirocumab is a protein. Alirocumab is expected to degrade to small peptides and individual amino acids. In clinical studies where alirocumab was administered in combination with atorvastatin or rosuvastatin, no relevant changes in statin concentrations were observed in the presence of repeated administration of alirocumab, indicating that cytochrome P450 enzymes (mainly CYP3A4 and CYP2C9) and transporter proteins such as P-gp and OATP were not affected by alirocumab.
Two elimination phases were observed for alirocumab. At low concentrations, the elimination is predominately through saturable binding to target (PCSK9), while at higher concentrations the elimination of alirocumab is largely through a non-saturable proteolytic pathway.
Based on a population pharmacokinetic analysis, the median apparent half-life of alirocumab at steady state was 17 to 20 days in patients receiving alirocumab at subcutaneous doses of 75 mg every 2 weeks or 150 mg every 2 weeks.
Specific Populations
A population pharmacokinetic analysis was conducted on data from 2799 patients. Age, body weight, gender, race, and creatinine clearance were found not to significantly influence alirocumab pharmacokinetics.
Pediatric Patients
The pharmacokinetics of alirocumab were evaluated in 140 pediatric patients aged 8 to 17 years with HeFH. Steady-state concentrations were reached at or before week 8 (first PK sampling during repeated dosing) with recommended dosing regimen [see Dosage and Administration (2.2)].
Renal Impairment
Since monoclonal antibodies are not known to be eliminated via renal pathways, renal function is not expected to impact the pharmacokinetics of alirocumab.
No data are available in patients with severe renal impairment.
Hepatic Impairment
Following administration of a single 75 mg SC dose, alirocumab pharmacokinetic profiles in patients with mild and moderate hepatic impairment were similar to those in patients with normal hepatic function.
No data are available in patients with severe hepatic impairment.
Drug-Drug Interactions
The median apparent half-life of alirocumab is reduced to 12 days when administered with a statin; however, this difference is not clinically meaningful.
12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the trials described below with the incidence of anti-drug antibodies in other trials, including those of PRALUENT or of other alirocumab products.
In adult patients with cardiovascular disease (Trial 1), the incidence of anti-alirocumab antibody (ADA) formation was 5.5% (504/9,091) in patients treated with PRALUENT 75 mg and/or 150 mg every 2 weeks for up to 5 years (with a median treatment exposure of 31 months). Neutralizing antibody (NAb) responses were observed in 0.5% (43/9,091) of all patients treated with PRALUENT. Of the patients who developed ADA, 8.5% (43/504) tested positive for NAb.
- While reductions in LDL-C were generally comparable in patients with or without ADA, including NAbs, some adult patients treated with PRALUENT with persistent or neutralizing antibodies experienced attenuation in LDL-C efficacy.
- Adult patients who developed ADA had a higher incidence of injection site reactions compared to patients without ADA (7.5% vs 3.6%) [see Adverse Reactions (6.1)].
In a pool of placebo-controlled and active-controlled trials of adult patients treated with PRALUENT 75 mg and/or 150 mg every 2 weeks as well as in a separate clinical trial of patients treated with PRALUENT 75 mg every 2 weeks or 300 mg every 4 weeks (including some patients with dose adjustment to 150 mg every 2 weeks), during the treatment period ranging from 6 to 24 months, the incidence of detecting ADA was 4.8% (147/3033) and NAb was 1.2% (36/3,033), which was similar to the results from the trial described above.
In pediatric patients aged 8 to 17 years with HeFH (Trial 12), the incidence of ADA for patients treated with PRALUENT was 3% (3/98) with a median treatment exposure of 24 weeks in patients receiving PRALUENT once every 2 weeks and 23 weeks in patients receiving PRALUENT once every 4 weeks with an optional up-titration. Of the 3 pediatric patients who developed ADA, no one tested positive for NAb.
Because of the low occurrence of ADA and the small number of pediatric patients enrolled, the effect of these antibodies on the pharmacokinetics, pharmacodynamics, safety, and/or effectiveness of PRALUENT in pediatric patients is unknown.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been conducted with alirocumab. The mutagenic potential of alirocumab has not been evaluated; however, monoclonal antibodies are not expected to alter DNA or chromosomes.
There were no adverse effects on surrogate markers of fertility (e.g., estrous cyclicity, testicular volume, ejaculate volume, sperm motility, or total sperm count per ejaculate) in a 6-month chronic toxicology study in sexually-mature monkeys subcutaneously administered at 5, 15, and 75 mg/kg/week at systemic exposures up to 103-fold the 150 mg every two weeks subcutaneous clinical dose based on serum AUC. In addition, there were no adverse alirocumab-related anatomic pathology or histopathology findings in reproductive tissues in rat or monkey toxicology studies at systemic exposures up to 11-fold and 103-fold respectively, in the 6-month studies, compared to clinical systemic exposure following a 150 mg every two weeks dose, based on serum AUC.
13.2 Animal Toxicology and/or Pharmacology
During a 13-week toxicology study of 75 mg/kg once weekly alirocumab in combination with 40 mg/kg once daily atorvastatin in adult monkeys, there were no effects of PRALUENT on the humoral immune response to keyhole limpet hemocyanin (KLH) after one to two months at exposures 100-fold greater than the exposure at the maximum recommended human dose of 150 mg every two weeks, based on AUC.
SPL UNCLASSIFIED SECTION
Manufactured by:
Regeneron Pharmaceuticals, Inc.
Tarrytown, NY 10591
U.S. License # 1760
PRALUENT is a registered trademark of Regeneron Pharmaceuticals, Inc.
© 2024 Regeneron Pharmaceuticals, Inc. All rights reserved.
SPL PATIENT PACKAGE INSERT SECTION
|
This Patient Information has been approved by the U.S. Food and Drug Administration. |
Revised: March 2024 | |||
|
Patient Information | ||||
|
What is PRALUENT?
It is not known if PRALUENT is safe and effective in children who are younger than 8 years of age or in children with other types of high cholesterol (hyperlipidemias). | ||||
|
Who should not use PRALUENT? | ||||
|
What should I tell my healthcare provider before using PRALUENT?
If you are pregnant during PRALUENT treatment, you are encouraged to call
Regeneron at 1-844-734-6643 to share information about the health of you and
your baby. | ||||
|
How should I use PRALUENT? *See the detailed "Instructions for Use" that comes with this Patient Information about the right way to prepare and give your PRALUENT injections.
| ||||
|
What are the possible side effects of PRALUENT? *allergic reactions. PRALUENT may cause allergic reactions that can be severe and require treatment in a hospital. Stop using PRALUENT and call your healthcare provider or go to the nearest hospital emergency room right away if you have any symptoms of an allergic reaction including: | ||||
|
|
| ||
|
The common side effects of PRALUENT include: | ||||
|
| |||
|
Tell your healthcare provider if you have any side effect that bothers you or
that does not go away. | ||||
|
General information about the safe and effective use of PRALUENT. | ||||
|
What are the ingredients in PRALUENT? | ||||
|
| |||
|
Manufactured by: Regeneron Pharmaceuticals, Inc. Tarrytown, NY 10591, U.S. License # 1760; PRALUENT is a registered trademark of Regeneron Pharmaceuticals, Inc. / ©2024 Regeneron Pharmaceuticals, Inc. All rights reserved. |
INSTRUCTIONS FOR USE SECTION
Instructions For Use
PRALUENT®** (PRAHL-u-ent)**
(alirocumab)
Injection, for Subcutaneous Injection
Single-Dose Pre-Filled Pen (150 mg/mL)
|
Important Information | |
|---|---|
|
|
|
Storage of PRALUENT
|
Keep this leaflet. If you have questions, ask your healthcare provider or call 1-844-PRALUENT (1-844-772-5836).
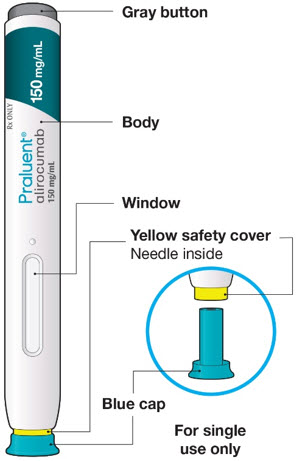
The parts of the PRALUENT pen are shown in this picture.
Step A: Getting ready for your injection.
Before you start you will need:
- the PRALUENT pen
- 1 alcohol wipe
- 1 cotton ball or gauze
- a sharps container or a puncture-resistant container (see Step B8)
A1: Look at the label on the pen.
- Check that you have the correct product and the correct dose.
- Check the expiration date (EXP):do not use if this date has passed.

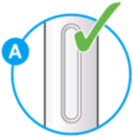
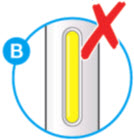
A2: Look at the window.
- Check the liquid is clear, colorless to pale yellow and free from particles (see Figure A).
- You may see air bubbles. This is normal. *Do not use if the window appears solid yellow (see Figure B). *Do not use this medicine if the solution is discolored or cloudy, or if it contains visible flakes or particles.
A3: Let the pen warm up at room temperature for 30 to 40 minutes.
- This is important for administering the entire dose and helps minimize discomfort.
- Take PRALUENT out of the refrigerator to warm up before using. *Do not heat the pen, let it warm up on its own. *Do not put the pen back in the refrigerator.
A4: Prepare the injection site.
- Wash your hands with soap and water and dry with a towel.
- Clean skin in the injection area with an alcohol wipe.
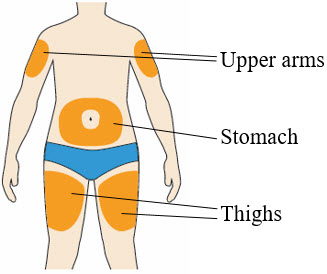
- You can inject into your (see picture):
- thighs
- stomach (except for the 2 inch area around your belly button)
- upper arms
- You can stand or sit to give yourself an injection.
Important:
- Change (rotate) your injection site each time you give yourself an injection. If you need to use the same injection site, make sure it is not the same spot on the site you used last time. *Do not inject into areas where the skin is tender, bruised, hard, or red.Do not inject PRALUENT into areas with visible veins, scars or stretch marks.
Step B: How to give your injection.
B1: After completing all steps in "Step A: Getting ready for your injection", pull off the blue cap.
*Do not pull off the cap until you are ready to inject. *Do not put the blue cap back on.
B2: Hold the PRALUENT pen like this.
*Do not touch the yellow safety cover.
- Make sure you can see the window.
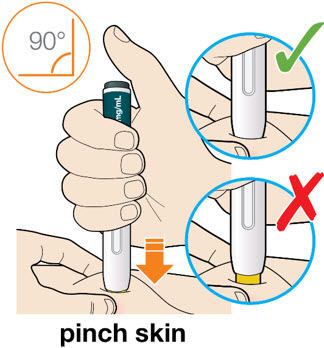
B3: Press the yellow safety cover on your skin at roughly a 90° angle.
*For children younger than 12 years of age, pinching the skin before and during the injection is required.
- In adults and children aged 12 years and older, pinching of skin may be required to make the injection site firm.
- Press and firmly hold the pen against your body until the yellow safety cover is no longer visible. The pen will not work if the yellow safety cover is not depressed fully.
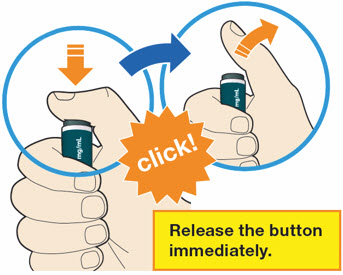
B4: Push and immediately release the gray button with your thumb.
- You will hear a click. Your injection has now started.
- The window will start to turn yellow.
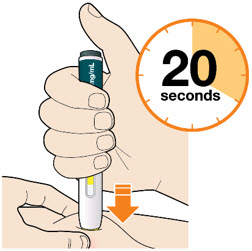
B5: Keep holding the pen against your skin after releasing the button.
- The injection may take up to 20 seconds.
B6: Check the window has turned yellow, before removing the pen.
*Do not remove the pen until the entire window has turned yellow.
- Your injection is complete when the window has turned completely yellow, you may hear a second click.
- If the window does not turn completely yellow, call 1-844-772-5836 for help.Do not give yourself a second dose without speaking to your healthcare provider.

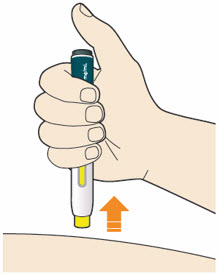
B7: Pull pen away from your skin.
*Do not rub the skin after the injection.
- If you see any blood, press a cotton ball or gauze on the site until the bleeding stops.
B8: Throw away (Discard) pen and cap.
*Do not put the blue cap back on.
- Throw away pen and cap in a puncture-resistant container immediately after they have been used.
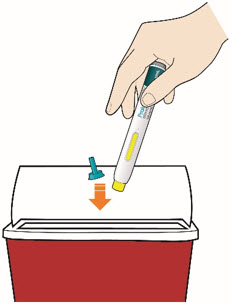
Disposing of used pens:
- Put your used pens in a FDA-cleared sharps disposal container right away after use.Do not throw away (dispose of) pens and caps in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
Keep PRALUENT and all medicines out of the reach of children.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Regeneron Pharmaceuticals, Inc.
Tarrytown, NY 10591
U.S. License # 1760
PRALUENT is a registered trademark of Regeneron Pharmaceuticals, Inc.
©2024 Regeneron Pharmaceuticals, Inc. All rights reserved.
Revised: March 2024
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
PRALUENT injection is a clear, colorless to pale yellow solution, supplied as follows:
|
Strength |
Package Size |
NDC |
|---|---|---|
|
75 mg/mL single-dose pre-filled pen |
1 pen |
61755-020-01 |
|
2 pens |
61755-020-02 | |
|
150 mg/mL single-dose pre-filled pen |
1 pen |
61755-021-01 |
|
2 pens |
61755-021-02 |
The needle shield is not made with natural rubber latex.
Store in a refrigerator at 36°F to 46°F (2°C to 8°C) in the original carton to protect from light. Do not freeze. Do not shake.
PRALUENT may be kept at room temperature up to 77°F (25°C) in the original carton for 30 days. If not used within the 30 days, discard PRALUENT.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Pregnancy
Advise women who are exposed to PRALUENT during pregnancy that there is a pregnancy safety study that monitors pregnancy outcomes. Encourage these patients to report their pregnancy to Regeneron at 1 844-734-6643 [see Use in Specific Populations (8.1)].
Hypersensitivity Reactions
Inform patients that serious hypersensitivity reactions (e.g., angioedema) have been reported in patients treated with PRALUENT. Advise patients on the symptoms of hypersensitivity reactions and instruct them to discontinue PRALUENT and seek medical attention promptly, if such symptoms occur.
Administration
Provide guidance to patients and caregivers on proper subcutaneous injection technique and how to use the pre-filled pen. Inform patients that it may take up to 20 seconds to inject PRALUENT. Inform patients the pre-filled pen should be allowed to warm to room temperature for 30 to 40 minutes prior to use if refrigerated.
