Dapsone
DAPSONETablets, USP25 mg & 100 mg
229af266-d901-43e2-828a-4931d1124ea3
HUMAN PRESCRIPTION DRUG LABEL
Sep 19, 2025
Seton Pharmaceuticals
DUNS: 828898002
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Dapsone
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
Dapsone
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
Drug Labeling Information
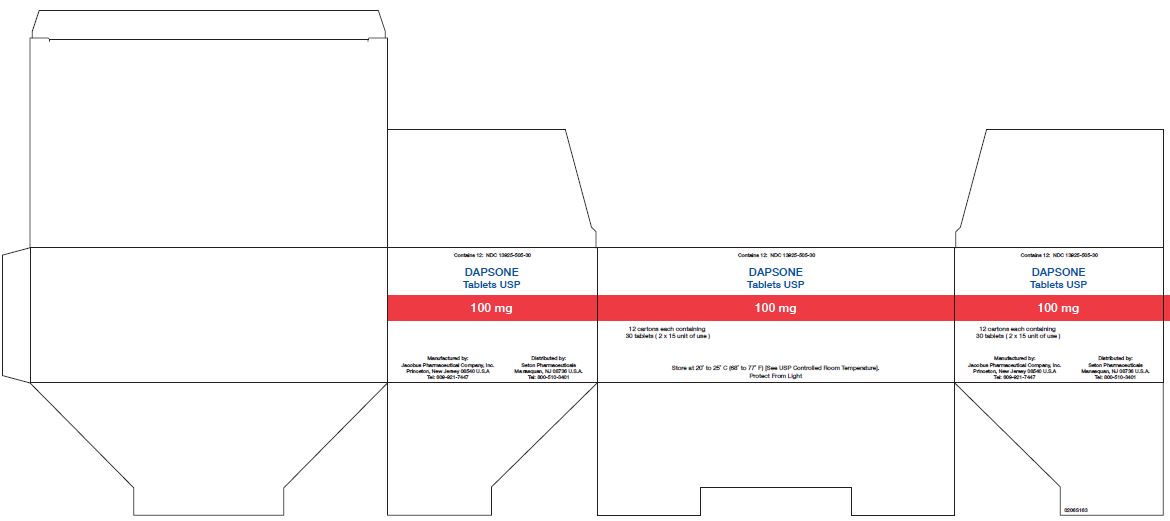
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Principal Display Panel - 100 mg 30 count
Contains 12: NDC 13925-505-30
Dapsone
Tablets USP
100 mg
12 cartons each containing
30 Tablets (2 x 15 unit of use)
Store at 20° to 25° C (68° to 77° F) [See USP Controlled Room Temperature].
Protect From Light
Principal Display Panel - 100 mg 30 count
NDC 13925-505-01
DAPSONE
Tablets, USP
100 mg
100 tablets
Caution: Federal law prohibits dispensing
without prescription. Dispense this product
in a well closed, light-resistant container
with child resistant closure.

DESCRIPTION SECTION
DESCRIPTION
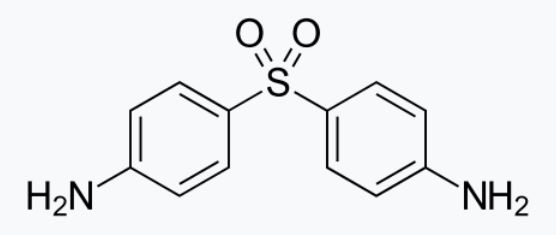
Dapsone-USP, 4,4'-diaminodiphenylsulfone (DDS), is a primary treatment for Dermatitis herpetiformis. It is an antibacterial drug for susceptible cases of leprosy. It is a white, odorless crystalline powder, practically in-soluble in water and insoluble in fixed and vegetable oils.
Dapsone is issued on prescription in tablets of 25 and 100 mg for oral use.
Inactive Ingredients: Colloidal silicone dioxide, magnesium stearate, microcrystalline cellulose and corn starch.
Dapsone-USP, 4,4'-diaminodiphenylsulfone (DDS), is a primary treatment for Dermatitis herpetiformis. It is an antibacterial drug for susceptible cases of leprosy. It is a white, odorless crystalline powder, practically in-soluble in water and insoluble in fixed and vegetable oils.
Dapsone is issued on prescription in tablets of 25 and 100 mg for oral use.

Inactive Ingredients: Colloidal silicone dioxide, magnesium stearate, microcrystalline cellulose and corn starch.
<p class="First">Dapsone-USP, 4,4'-diaminodiphenylsulfone (DDS), is a primary treatment for Dermatitis herpetiformis. It is an antibacterial drug for susceptible cases of leprosy. It is a white, odorless crystalline powder, practically in-soluble in water and insoluble in fixed and vegetable oils.</p><p>Dapsone is issued on prescription in tablets of 25 and 100 mg for oral use.</p><div class="Figure"><img id="mm01" src="/validator- lite/validator/spl/2d014f94-1f55-4f1c-8152-469a5bdb4f02/image-1-dapsone- structure.jpg" alt="" data-mce- src="../validator/spl/2d014f94-1f55-4f1c-8152-469a5bdb4f02/image-1-dapsone- structure.jpg"></div><p>Inactive Ingredients: Colloidal silicone dioxide, magnesium stearate, microcrystalline cellulose and corn starch.</p>
