ZAVZPRET
These highlights do not include all the information needed to use ZAVZPRET safely and effectively. See full prescribing information for ZAVZPRET. ZAVZPRET™ (zavegepant) nasal spray Initial U.S. Approval: 2023
1191475f-e999-49f4-a805-93ad08596b1b
HUMAN PRESCRIPTION DRUG LABEL
Mar 9, 2023
U.S. Pharmaceuticals
DUNS: 829076905
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
zavegepant
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL – Nasal Spray Carton Sample
PROFESSIONAL
SAMPLE -
NOT FOR SALE
NDC 63539-135-02
Rx only
ZavzpretTM
(zavegepant)
nasal spray 10 mg
This box contains:
• 1 unit-dose nasal spray device
containing 10 mg of zavegepant.
• 1 Prescribing Information
booklet.
• 1 Instructions for Use Leaflet.
For Intranasal use only.
1 spray (10 mg dose) per unit.
Do not test spray, prime
or press the plunger
before use.

ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are discussed in greater detail in other sections of the labeling:
•
Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety of ZAVZPRET for the acute treatment of migraine in adults has been evaluated in two randomized, double-blind, placebo-controlled trials (Study 1 and Study 2) in patients with migraine who received one 10 mg dose of ZAVZPRET nasal spray (N=1023) or placebo (N=1056) [see Clinical Studies (14)]. Approximately 83% were female, 81% were White, 20% were Hispanic or Latino, and 15% were Black. The mean age at study entry was 41 years (range 18-79 years of age).
Adverse reactions in Study 1 and 2 are shown in Table 1.
Table 1: Adverse Reactions Occurring in At Least 2% of Patients Treated with ZAVZPRET and at a Frequency Greater than Placebo in Study 1 and 2
| ||
|
Adverse Reaction |
ZAVZPRET N=1023 % |
Placebo N=1056 % |
|
Taste Disorders* |
18 |
4 |
|
Nausea |
4 |
1 |
|
Nasal Discomfort |
3 |
1 |
|
Vomiting |
2 |
<1 |
Hypersensitivity, including facial swelling and urticaria, occurred in less than 1% of patients treated with ZAVZPRET [see Contraindications (4) and Warnings and Precautions (5.1)].
Long-term safety was assessed in an open-label extension study. That study evaluated 603 patients, dosing intermittently for up to one year, including 360 patients who were exposed to ZAVZPRET 10 mg for at least 6 months, and 298 who were exposed for at least one year, all of whom treated an average of at least two migraine attacks per month.
Most common adverse reactions (at least 2% of patients treated with ZAVZPRET and greater than placebo) were taste disorders, nausea, nasal discomfort, and vomiting. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Pfizer Inc. at 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 OATP1B3 or NTCP Inhibitors
Concomitant administration of ZAVZPRET with inhibitors of the organic anion transporting polypeptide 1B3 (OATP1B3) or sodium taurocholate co-transporting polypeptide (NTCP) transporters may result in a significant increase in zavegepant exposure. Avoid concomitant administration of ZAVZPRET with drugs that inhibit OATP1B3 or NTCP transporters [see Clinical Pharmacology (12.3)].
7.2 OATP1B3 or NTCP Inducers
Concomitant administration of ZAVZPRET with inducers of OATP1B3 or NTCP transporters may result in a decrease in zavegepant exposure. Avoid concomitant administration of ZAVZPRET with drugs that induce OATP1B3 or NTCP transporters [see Clinical Pharmacology (12.3)].
7.3 Intranasal Decongestants
Concomitant administration of ZAVZPRET with intranasal decongestants may decrease the absorption of zavegepant. Avoid concomitant administration of intranasal decongestants with ZAVZPRET. When concomitant use is unavoidable, intranasal decongestants should be administered at least 1 hour after ZAVZPRET administration [see Clinical Pharmacology (12.3)].
•
Avoid use with drugs that inhibit OATP1B3 or NTCP transporters. (7.1)
•
Avoid use with drugs that induce OATP1B3 or NTCP transporters. (7.2)
•
Avoid use of intranasal decongestants; if unavoidable, administer intranasal decongestants at least 1 hour after ZAVZPRET administration. (7.3)
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Information
The recommended dose of ZAVZPRET is 10 mg given as a single spray in one nostril, as needed.
The maximum dose that may be given in a 24-hour period is 10 mg (one spray). The safety of treating more than 8 migraines in a 30-day period has not been established.
•
The recommended dose is 10 mg given as a single spray in one nostril, as needed. (2.1)
•
The maximum dose in a 24-hour period is 10 mg (one spray). (2.1)
•
The safety of treating more than 8 migraines in a 30-day period has not been established. (2.1)
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Intranasal administration of zavegepant (0, 0.3, 0.8, or 2.5 mg/day) to Tg.rasH2 mice for 26 weeks resulted in no evidence of drug-related tumors.
Intranasal administration of zavegepant (0, 2, 9, or 18.8 mg/kg/day) to rats for up to 96 weeks resulted in no evidence of drug-related tumors. Plasma exposure (AUC) at the highest dose tested was approximately 140 times that in humans at the maximum recommended human dose (MRHD) of 10 mg/day.
Mutagenesis
Zavegepant was negative in in vitro (bacterial reverse mutation, chromosomal aberration in Chinese hamster ovary cells) and in vivo (rat micronucleus) assays.
Impairment of Fertility
Subcutaneous administration of zavegepant (0, 5, 15, or 25 mg/kg/day) to male and female rats prior to and during mating and continuing in females to gestation day 7 resulted in no adverse effects on fertility or reproductive performance. Plasma exposures (AUC) at the highest dose tested were approximately 2800 times that in humans at MRHD.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
The efficacy of ZAVZPRET for the acute treatment of migraine with or without aura in adults was demonstrated in two randomized, double-blind, placebo- controlled trials (Study 1 and Study 2). In both studies, patients were instructed to treat a migraine of moderate to severe headache pain intensity. Rescue medication (i.e., NSAIDs, acetaminophen, and/or an antiemetic) was allowed 2 hours after the initial treatment. Other forms of rescue medication such as triptans were not allowed within 48 hours of initial treatment. In Study 1 and Study 2, 13.4% and 13.6% of patients were taking preventive medications for migraine at baseline, respectively. None of the patients were on concomitant preventive medication that act on the CGRP pathway.
In Study 1 (NCT04571060), patients were randomized to receive a single dose of ZAVZPRET 10 mg (N=623) or placebo (N=646). Efficacy was demonstrated with ZAVZPRET 10 mg by an effect on the coprimary endpoints of pain freedom and most bothersome symptom (MBS) freedom at 2 hours after a single dose, compared to placebo. Pain freedom was defined as a reduction of moderate or severe headache pain to no headache pain, and MBS freedom was defined as the absence of the self-identified MBS (i.e., photophobia, phonophobia, or nausea). The most common MBS reported before dosing was photophobia (55%), followed by nausea (28%), and phonophobia (16%).
In Study 1, the percentage of patients achieving headache pain freedom and MBS freedom 2 hours after a single dose was statistically significantly greater in patients who received ZAVZPRET compared to those who received placebo (Table 2).
Table 2: Efficacy Endpoints in Study 1
| ||
|
ZAVZPRET 10 mg |
Placebo | |
|
Pain Free at 2 hours | ||
|
n/N* |
147/623 |
96/646 |
|
% Responders |
23.6 |
14.9 |
|
Difference from placebo (%) |
8.8 | |
|
p-value |
<0.001 | |
|
MBS†** Free at 2 hours** | ||
|
n/N* |
247/623 |
201/646 |
|
% Responders |
39.6 |
31.1 |
|
Difference from placebo (%) |
8.7 | |
|
p-value |
0.001 |
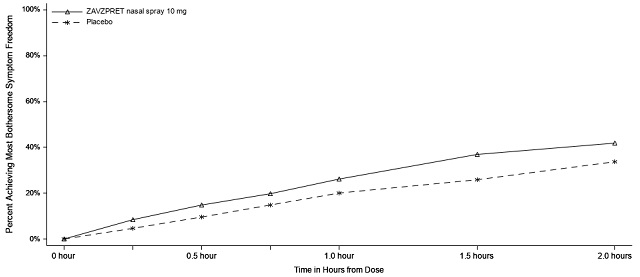
Figures 1 and 2 present the percentage of patients achieving migraine pain freedom and MBS freedom within 2 hours following treatment in Study 1.
Figure 1: Percentage of Patients Achieving Pain Freedom within 2 Hours in Study 1
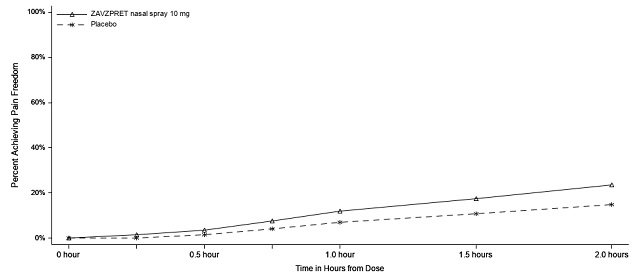
Figure 2: Percentage of Patients Achieving MBS Freedom within 2 Hours in Study 1
In Study 1, statistically significant effects of ZAVZPRET compared to placebo were demonstrated for the additional efficacy endpoints of pain relief at 2 hours post-dose, return to normal function at 2 hours post-dose, sustained pain freedom from 2 to 48 hours post-dose (Table 3), and phonophobia and photophobia freedom at 2 hours post-dose. Pain relief was defined as a reduction in migraine pain from moderate or severe severity to mild or none. The measurement of the percentage of patients reporting normal function at two hours after dosing was derived from a single item questionnaire, asking patients to select one response on a 4-point scale: normal function, mild impairment, severe impairment, or required bedrest.
Table 3: Additional Efficacy Endpoints in Study 1
| ||
|
ZAVZPRET 10 mg |
Placebo | |
|
Pain Relief at 2 hours | ||
|
n/N* |
366/623 |
321/646 |
|
% Responders |
58.7 |
49.7 |
|
Difference from placebo (%) |
9.0 | |
|
p-value |
0.001 | |
|
Percentage of Patients Reporting Normal Function at 2 hours† | ||
|
n/N* |
204/570 |
152/593 |
|
% Responders |
35.8 |
25.6 |
|
Difference from placebo (%) |
10.2 | |
|
p-value |
<0.001 | |
|
Sustained Pain Freedom from 2 to 48 hours | ||
|
n/N* |
77/623 |
56/646 |
|
% Responders |
12.4 |
8.7 |
|
Difference from placebo (%) |
3.7 | |
|
p-value |
0.031 |
The incidence of photophobia and phonophobia was reduced following administration of ZAVZPRET 10 mg as compared to placebo.
In Study 2 (NCT03872453), patients were randomized to receive a single dose of ZAVZPRET 10 mg (n=391) or placebo (n=401).
In Study 2, statistically significant efficacy was demonstrated with ZAVZPRET 10 mg by an effect on the coprimary endpoints of pain freedom and most bothersome symptom (MBS) freedom at 2 hours after a single dose, compared to placebo. Pain freedom was observed in 22.5% of patients receiving ZAVZPRET and 15.5% of patients receiving placebo (p-value = 0.011). MBS freedom was observed in 41.9% of patients receiving ZAVZPRET and 33.7% of patients receiving placebo (p-value = 0.016). The most common MBS reported before dosing was photophobia (53%), followed by nausea (31%), and phonophobia (15%).
Table 4: Efficacy Endpoints in Study 2
| ||
|
ZAVZPRET 10 mg |
Placebo | |
|
Pain Free at 2 hours | ||
|
n/N* |
88/391 |
62/401 |
|
% Responders |
22.5 |
15.5 |
|
Difference from placebo (%) |
7.0 | |
|
p-value |
0.011 | |
|
MBS†** Free at 2 hours** | ||
|
n/N* |
164/391 |
135/401 |
|
% Responders |
41.9 |
33.7 |
|
Difference from placebo (%) |
8.3 | |
|
p-value |
0.016 |
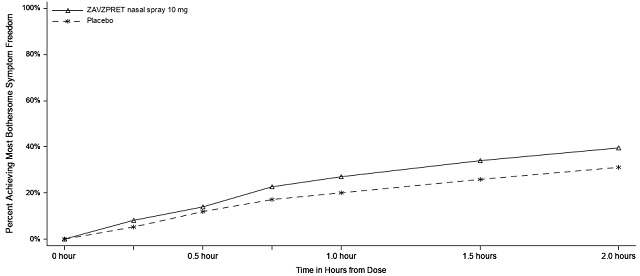
Figure 3: Percentage of Patients Achieving Pain Freedom within 2 Hours in Study 2
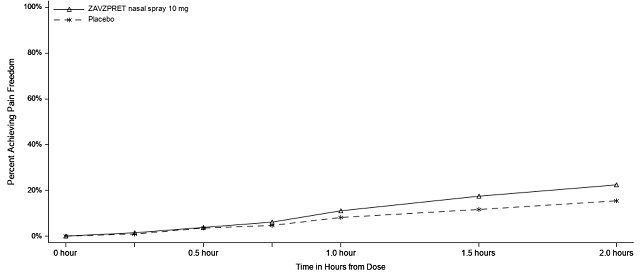
Figure 4: Percentage of Patients Achieving MBS Freedom within 2 Hours in Study 2