Oxandrolone
Oxandrolone Tablet, USP
7127ac16-f673-4b66-b5e5-5f881c85aa39
HUMAN PRESCRIPTION DRUG LABEL
Apr 29, 2022
Bryant Ranch Prepack
DUNS: 171714327
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
oxandrolone
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
Drug Labeling Information
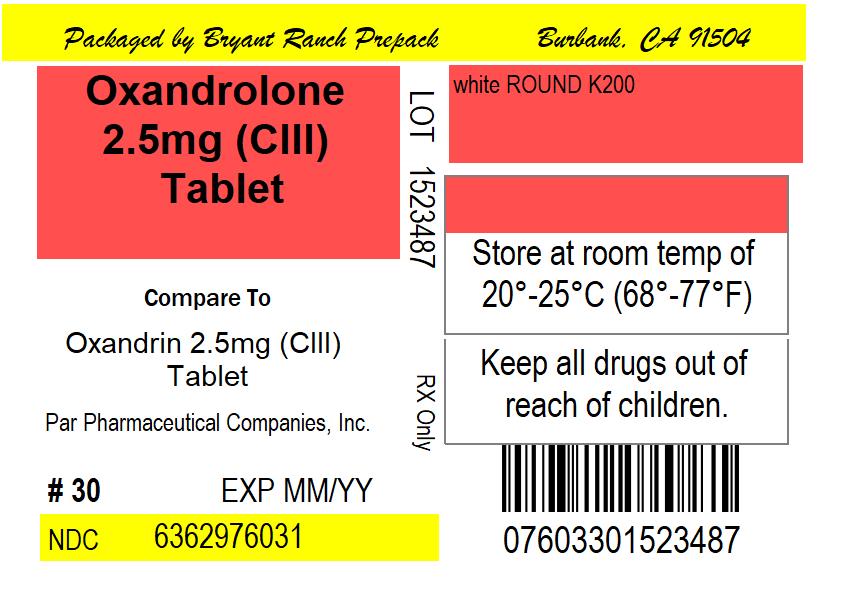
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Oxandrolone 2.5mg (CIII) Tablet
BOXED WARNING SECTION
BOXED WARNINGS
PELIOSIS HEPATIS, A CONDITION IN WHICH LIVER AND SOMETIMES SPLENIC TISSUE IS REPLACED WITH BLOOD-FILLED CYSTS, HAS BEEN REPORTED IN PATIENTS RECEIVING ANDROGENIC ANABOLIC STEROID THERAPY. THESE CYSTS ARE SOMETIMES PRESENT WITH MINIMAL HEPATIC DYSFUNCTION, BUT AT OTHER TIMES THEY HAVE BEEN ASSOCIATED WITH LIVER FAILURE. THEY ARE OFTEN NOT RECOGNIZED UNTIL LIFE-THREATENING LIVER FAILURE OR INTRA-ABDOMINAL HEMORRHAGE DEVELOPS. WITHDRAWAL OF DRUG USUALLY RESULTS IN COMPLETE DISAPPEARANCE OF LESIONS. LIVER CELL TUMORS ARE ALSO REPORTED. MOST OFTEN THESE TUMORS ARE BENIGN AND ANDROGEN-DEPENDENT, BUT FATAL MALIGNANT TUMORS HAVE BEEN REPORTED. WITHDRAWAL OF DRUG OFTEN RESULTS IN REGRESSION OR CESSATION OF PROGRESSION OF THE TUMOR. HOWEVER, HEPATIC TUMORS ASSOCIATED WITH ANDROGENS OR ANABOLIC STEROIDS ARE MUCH MORE VASCULAR THAN OTHER HEPATIC TUMORS AND MAY BE SILENT UNTIL LIFE-THREATENING INTRA-ABDOMINAL HEMORRHAGE DEVELOPS. BLOOD LIPID CHANGES THAT ARE KNOWN TO BE ASSOCIATED WITH INCREASED RISK OF ATHEROSCLEROSIS ARE SEEN IN PATIENTS TREATED WITH ANDROGENS OR ANABOLIC STEROIDS. THESE CHANGES INCLUDE DECREASED HIGH-DENSITY LIPOPROTEINS AND SOMETIMES INCREASED LOW-DENSITY LIPOPROTEINS. THE CHANGES MAY BE VERY MARKED AND COULD HAVE A SERIOUS IMPACT ON THE RISK OF ATHEROSCLEROSIS AND CORONARY ARTERY DISEASE.
INDICATIONS & USAGE SECTION
INDICATIONS AND USAGE
Oxandrolone is indicated as adjunctive therapy to offset the protein catabolism associated with prolonged administration of corticosteroids, and for the relief of the bone pain frequently accompanying osteoporosis (see DOSAGE AND ADMINISTRATION).
CONTRAINDICATIONS SECTION
CONTRAINDICATIONS
- Known or suspected carcinoma of the prostate or the male breast.
- Carcinoma of the breast in females with hypercalcemia (androgenic anabolic steroids may stimulate osteolytic bone resorption).
- Pregnancy, because of possible masculinization of the fetus. Oxandrolone has been shown to cause embryotoxicity, fetotoxicity, infertility, and masculinization of female animal offspring when given in doses 9 times the human dose.
- Nephrosis, the nephrotic phase of nephritis.
- Hypercalcemia.
ADVERSE REACTIONS SECTION
ADVERSE REACTIONS
Patients with moderate to severe COPD or COPD patients who are unresponsive to bronchodilators should be monitored closely for COPD exacerbation and fluid retention.
The following adverse reactions have been associated with use of anabolic steroids:
Hepatic
Cholestatic jaundice with, rarely, hepatic necrosis and death. Hepatocellular neoplasms and peliosis hepatis with long-term therapy (seeWARNINGS). Reversible changes in liver function tests also occur including increased bromsulfophthalein (BSP) retention, changes in alkaline phosphatase and increases in serum bilirubin, aspartate aminotransferase (AST, SGOT) and alanine aminotransferase (ALT, SGPT).
In Males
Prepubertal:
Phallic enlargement and increased frequency or persistence of erections.
Postpubertal:
Inhibition of testicular function, testicular atrophy and oligospermia, impotence, chronic priapism, epididymitis, and bladder irritability.
In Females
Clitoral enlargement, menstrual irregularities.
CNS
Habituation, excitation, insomnia, depression, and changes in libido.
Hematologic
Bleeding in patients on concomitant anticoagulant therapy.
Breast
Gynecomastia.
Larynx
Deepening of the voice in females.
Hair
Hirsutism and male pattern baldness in females.
Skin
Acne (especially in females and prepubertal males).
Skeletal
Premature closure of epiphyses in children (seePRECAUTIONS, Pediatric Use).
Fluid and Electrolytes
Edema, retention of serum electrolytes (sodium chloride, potassium, phosphate, calcium).
Metabolic/Endocrine
Decreased glucose tolerance (seePRECAUTIONS, Laboratory Tests), increased creatinine excretion, increased serum levels of creatinine phosphokinase (CPK). Masculinization of the fetus. Inhibition of gonadotropin secretion.
DESCRIPTION SECTION
DESCRIPTION
Oxandrolone oral tablets contain 2.5 mg or 10 mg of the anabolic steroid oxandrolone. Oxandrolone is 17β-hydroxy-17α-methyl-2-oxa-5α-androstan-3-one with the following structural formula:

Molecular Formula: C19H30O3
Molecular Weight: 306.44
Inactive ingredients include: corn starch, lactose monohydrate, magnesium stearate, and hypromellose.
Meets USP Dissolution Test 2.
CLINICAL PHARMACOLOGY SECTION
CLINICAL PHARMACOLOGY
Anabolic steroids are synthetic derivatives of testosterone. Certain clinical effects and adverse reactions demonstrate the androgenic properties of this class of drugs. Complete dissociation of anabolic and androgenic effects has not been achieved. The actions of anabolic steroids are therefore similar to those of male sex hormones with the possibility of causing serious disturbances of growth and sexual development if given to young children. Anabolic steroids suppress the gonadotropic functions of the pituitary and may exert a direct effect upon the testes.
During exogenous administration of anabolic androgens, endogenous testosterone release is inhibited through inhibition of pituitary luteinizing hormone (LH). At large doses, spermatogenesis may be suppressed through feedback inhibition of pituitary follicle-stimulating hormone (FSH).
Anabolic steroids have been reported to increase low-density lipoproteins and decrease high-density lipoproteins. These levels revert to normal on discontinuation of treatment.
DRUG ABUSE AND DEPENDENCE SECTION
DRUG ABUSE AND DEPENDENCE
Oxandrolone is classified as a controlled substance under the Anabolic Steroids Control Act of 1990 and has been assigned to Schedule III (non- narcotic).
WARNINGS SECTION
WARNINGS
Cholestatic hepatitis and jaundice may occur with 17-alpha-alkylated androgens at a relatively low dose. If cholestatic hepatitis with jaundice appears or if liver function tests become abnormal, oxandrolone should be discontinued and the etiology should be determined. Drug-induced jaundice is reversible when the medication is discontinued.
In patients with breast cancer, anabolic steroid therapy may cause hypercalcemia by stimulating osteolysis. Oxandrolone therapy should be discontinued if hypercalcemia occurs.
Edema with or without congestive heart failure may be a serious complication in patients with pre-existing cardiac, renal, or hepatic disease. Concomitant administration of adrenal cortical steroid or ACTH may increase the edema.
In children, androgen therapy may accelerate bone maturation without producing compensatory gain in linear growth. This adverse effect results in compromised adult height. The younger the child, the greater the risk of compromising final mature height. The effect on bone maturation should be monitored by assessing bone age of the left wrist and hand every 6 months (see PRECAUTIONS, Laboratory****Tests).
Geriatric patients treated with androgenic anabolic steroids may be at an increased risk for the development of prostatic hypertrophy and prostatic carcinoma. ANABOLIC STEROIDS HAVE NOT BEEN SHOWN TO ENHANCE ATHLETIC ABILITY.
PRECAUTIONS SECTION
PRECAUTIONS
Concurrent dosing of oxandrolone and warfarin may result in unexpectedly large increases in the International Normalized Ratio (INR) or prothrombin time (PT). When oxandrolone is prescribed to patients being treated with warfarin, doses of warfarin may need to be decreased significantly to maintain the desirable INR level and diminish the risk of potentially serious bleeding (seePRECAUTIONS, Drug Interactions**).**
General
Women should be observed for signs of virilization (deepening of the voice, hirsutism, acne, clitoromegaly). Discontinuation of drug therapy at the time of evidence of mild virilism is necessary to prevent irreversible virilization. Some virilizing changes in women are irreversible even after prompt discontinuance of therapy and are not prevented by concomitant use of estrogens. Menstrual irregularities may also occur.
Anabolic steroids may cause suppression of clotting factors II, V, VII, and X, and an increase in prothrombin time.
Information for Patients
The physician should instruct patients to report immediately any use of warfarin and any bleeding.
The physician should instruct patients to report any of the following side effects of androgens:
Males:
Too frequent or persistent erections of the penis, appearance or aggravation of acne.
Females:
Hoarseness, acne, changes in menstrual periods, or more facial hair.
All patients:
Nausea, vomiting, changes in skin color, or ankle swelling.
Geriatric Use:
Certain geriatric use information is protected by marketing exclusivity.
Laboratory Tests
Women with disseminated breast carcinoma should have frequent determination of urine and serum calcium levels during the course of therapy (see WARNINGS).
Because of the hepatotoxicity associated with the use of 17-alpha-alkylated androgens, liver function tests should be obtained periodically.
Periodic (every 6 months) x-ray examinations of bone age should be made during treatment of children to determine the rate of bone maturation and the effects of androgen therapy on the epiphyseal centers.
Androgenic anabolic steroids have been reported to increase low-density lipoproteins and decrease high-density lipoproteins. Therefore, caution is required when administering these agents to patients with a history of cardiovascular disease or who are at risk for cardiovascular disease. Serum determination of lipid levels should be performed periodically and therapy adjusted accordingly.
Hemoglobin and hematocrit should be checked periodically for polycythemia in patients who are receiving high doses of anabolic steroids.
Drug Interactions
Anticoagulants:
Anabolic steroids may increase sensitivity to oral anticoagulants. Dosage of the anticoagulant may have to be decreased in order to maintain desired prothrombin time. Patients receiving oral anticoagulant therapy require close monitoring, especially when anabolic steroids are started or stopped.
Warfarin:
A multidose study of oxandrolone, given as 5 or 10 mg BID in 15 healthy subjects concurrently treated with warfarin, resulted in a mean increase in S-warfarin half-life from 26 to 48 hours and AUC from 4.55 to 12.08 ng•hr/mL; similar increases in R-warfarin half-life and AUC were also detected. Microscopic hematuria (9/15) and gingival bleeding (1/15) were also observed. A 5.5-fold decrease in the mean warfarin dose from 6.13 mg/day to 1.13 mg/day (approximately 80-85% reduction of warfarin dose), was necessary to maintain a target INR of 1.5. When oxandrolone therapy is initiated in a patient already receiving treatment with warfarin, the INR or prothrombin time (PT) should be monitored closely and the dose of warfarin adjusted as necessary until a stable target INR or PT has been achieved.
Furthermore, in patients receiving both drugs, careful monitoring of the INR or PT, and adjustment of the warfarin dosage if indicated are recommended when the oxandrolone dose is changed or discontinued. Patients should be closely monitored for signs and symptoms of occult bleeding.
Oral Hypoglycemic Agents:
Oxandrolone may inhibit the metabolism of oral hypoglycemic agents.
Adrenal Steroids or ACTH:
In patients with edema, concomitant administration with adrenal cortical steroids or ACTH may increase the edema.
Drug/Laboratory Test Interactions
Anabolic steroids may decrease levels of thyroxine-binding globulin, resulting in decreased total T4 serum levels and increased resin uptake of T3 and T4. Free thyroid hormone levels remain unchanged. In addition, a decrease in PBI and radioactive iodine uptake may occur.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Animal Data:
Oxandrolone has not been tested in laboratory animals for carcinogenic or mutagenic effects. In 2-year chronic oral rat studies, a dose-related reduction of spermatogenesis and decreased organ weights (testes, prostate, seminal vesicles, ovaries, uterus, adrenals, and pituitary) were shown.
Human Data:
Liver cell tumors have been reported in patients receiving long-term therapy with androgenic anabolic steroids in high doses (see WARNINGS). Withdrawal of the drugs did not lead to regression of the tumors in all cases.
Geriatric patients treated with androgenic anabolic steroids may be at an increased risk for the development of prostatic hypertrophy and prostatic carcinoma.
Pregnancy
Teratogenic effects-Pregnancy Category X (seeCONTRAINDICATIONS).
Nursing Mothers
It is not known whether anabolic steroids are excreted in human milk. Because of the potential of serious adverse reactions in nursing infants from oxandrolone, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Anabolic agents may accelerate epiphyseal maturation more rapidly than linear growth in children and the effect may continue for 6 months after the drug has been stopped. Therefore, therapy should be monitored by x-ray studies at 6-month intervals in order to avoid the risk of compromising adult height. Androgenic anabolic steroid therapy should be used very cautiously in children and only by specialists who are aware of the effects on bone maturation (see WARNINGS).
OVERDOSAGE SECTION
OVERDOSAGE
No symptoms or signs associated with overdosage have been reported. It is possible that sodium and water retention may occur.
The oral LD50 of oxandrolone in mice and dogs is greater than 5,000 mg/kg. No specific antidote is known, but gastric lavage may be used.
DOSAGE & ADMINISTRATION SECTION
DOSAGE AND ADMINISTRATION
Therapy with anabolic steroids is adjunctive to and not a replacement for conventional therapy. The duration of therapy with oxandrolone will depend on the response of the patient and the possible appearance of adverse reactions. Therapy should be intermittent.
Adults
The response of individuals to anabolic steroids varies. The daily adult dosage is 2.5 mg to 20 mg given in 2 to 4 divided doses. The desired response may be achieved with as little as 2.5 mg or as much as 20 mg daily. A course of therapy of 2 to 4 weeks is usually adequate. This may be repeated intermittently as indicated.
Children
For children the total daily dosage of oxandrolone is <0.1 mg per kilogram body weight or <0.045 mg per pound of body weight. This may be repeated intermittently as indicated.
HOW SUPPLIED SECTION
HOW SUPPLIED
NDC: 63629-7603-1: 30 Tablets in a BOTTLE
NDC: 63629-7603-2: 100 Tablets in a BOTTLE
NDC: 63629-7603-3: 90 Tablets in a BOTTLE
