Suprane
These highlights do not include all the information needed to use SUPRANE safely and effectively. See full prescribing information for SUPRANE. SUPRANE (desflurane) liquid, for inhalation useInitial U.S. Approval: 1992
efbb228b-7b38-4176-8bae-826103178c35
HUMAN PRESCRIPTION DRUG LABEL
Feb 23, 2023
Baxter Healthcare Corporation
DUNS: 005083209
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
DESFLURANE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (1)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABELING - PRINCIPAL DISPLAY PANEL

Container Label
NDC 10019-644-64** Rx ONLY**
Suprane
(desflurane, USP)
Liquid for Inhalation
240 mL
Manufactured by:
Baxter Healthcare of Puerto Rico
Guayama, Puerto Rico 00784 USA
NOVAPLUS Logo
(±) 1,2,2,2 - tetrafluoroethyl
difluoromethyl ether
A nonflammable, nonexplosive
inhalation anesthetic
Store at room temperature
15°-30°C(59°-86°F).
Replace cap after each use.
**IMPORTANT:**See package insert for
dosage and directions for use.
For Product Inquiry 1 800 ANA DRUG
(1-800-262-3784)
Suprane is a trademark of
Baxter International Inc.
NOVAPLUS is a registered trademark of
Novation, LLC
07-09-69-536
N3 10019 64464 6
Lot
EXP. DATE
MFG. DATE
RECENT MAJOR CHANGES SECTION
RECENT MAJOR CHANGES
Contraindications (4) 11/2022
Warnings and Precautions; Malignant Hyperthermia (5.1) 11/2022
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate and well-controlled studies in pregnant women. In animal reproduction studies, embryo-fetal toxicity (reduced viable fetuses and/or increased post-implantation loss) was noted in pregnant rats and rabbits administered 1 MAC desflurane for 4 hours a day (4 MAC-hours/day) during organogenesis.
Published studies in pregnant primates demonstrate that the administration of anesthetic and sedation drugs that block NMDA receptors and/or potentiate GABA activity during the period of peak brain development increases neuronal apoptosis in the developing brain of the offspring when used for longer than 3 hours. There are no data on pregnancy exposures in primates corresponding to periods prior to the third trimester in humans [See Data].
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15- 20%, respectively.
Clinical Considerations
Labor or Delivery
The safety of SUPRANE during labor or delivery has not been demonstrated. SUPRANE is a uterine-relaxant.
Data
Animal Data
Pregnant rats were exposed to 8.2% desflurane (1 MAC; 60% oxygen) for 0.5, 1.0, or 4.0 hours (0.5, 1.0, or 4.0 MAC-hours) per day during organogenesis (Gestation Day 6-15).
Embryo-fetal toxicity (increased post-implantation loss and reduced viable fetuses) was noted in the 4 hour treatment group in the presence of maternal toxicity (reduced body weight gain). There was no evidence of malformations in any group.
Pregnant rabbits were exposed to 8.9% desflurane (1 MAC; 60% oxygen) for 0.5, 1.0, or 3.0 hours per day during organogenesis (Gestation Days 6-18). Fetal toxicity (reduced viable fetuses) was noted in the 3 hour treatment group in the presence of maternal toxicity (reduced body weight). There was no evidence of malformations in any group.
Pregnant rats were exposed to 8.2% desflurane (1 MAC; 60% oxygen) for 0.5, 1.0, or 4.0 hours per day from late gestation and through lactation (Gestation Day 15 to Lactation Day 21). Pup body weights were reduced in the 4 hours per day group in the presence of maternal toxicity (increased mortality and reduced body weight gain). This study did not evaluate neurobehavioral function including learning and memory or reproductive behavior in the first generation (F1) pups.
In a published study in primates, administration of an anesthetic dose of ketamine for 24 hours on Gestation Day 122 increased neuronal apoptosis in the developing brain of the fetus. In other published studies, administration of either isoflurane or propofol for 5 hours on Gestation Day 120 resulted in increased neuronal and oligodendrocyte apoptosis in the developing brain of the offspring. With respect to brain development, this time period corresponds to the third trimester of gestation in the human. The clinical significance of these findings is not clear; however, studies in juvenile animals suggest neuroapoptosis correlates with long-term cognitive deficits [See Warnings and Precautions (5.6), Use in Specific Populations (8.4), and Nonclinical Toxicology (13.2)].
8.2 Lactation
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when SUPRANE is administered to a nursing woman.
8.4 Pediatric Use
Respiratory Adverse Reactions in Pediatric Patients
SUPRANE is indicated for maintenance of anesthesia in infants and children after induction of anesthesia with agents other than SUPRANE, and tracheal intubation.
Is not approved for maintenance of anesthesia in non-intubated children due to an increased incidence of respiratory adverse reactions, including coughing (26%), laryngospasm (13%) and secretions (12%) [See Clinical Studies (14.5)].
Children, particularly if 6 years old or younger, who are under an anesthetic maintenance of SUPRANE delivered via laryngeal mask airway (LMA™ mask) are at increased risk for adverse respiratory reactions, e.g., coughing and laryngospasm, especially with removal of the laryngeal mask airway under deep anesthesia [See Clinical Studies (14.5)]. Therefore, closely monitor these patients for signs and symptoms associated with laryngospasm and treat accordingly.
When SUPRANE is used for maintenance of anesthesia in children with asthma or a history of recent upper airway infection, there is an increased risk for airway narrowing and increases in airway resistance. Therefore, closely monitor these patients for signs and symptoms associated with airway narrowing and treat accordingly.
Published juvenile animal studies demonstrate that the administration of anesthetic and sedation drugs, such as SUPRANE, that either block NMDA receptors or potentiate the activity of GABA during the period of rapid brain growth or synaptogenesis, results in widespread neuronal and oligodendrocyte cell loss in the developing brain and alterations in synaptic morphology and neurogenesis. Based on comparisons across species, the window of vulnerability to these changes is believed to correlate with exposures in the third trimester of gestation through the first several months of life, but may extend out to approximately 3 years of age in humans.
In primates, exposure to 3 hours of ketamine that produced a light surgical plane of anesthesia did not increase neuronal cell loss, however, treatment regimens of 5 hours or longer of isoflurane increased neuronal cell loss. Data from isoflurane-treated rodents and ketamine-treated primates suggest that the neuronal and oligodendrocyte cell losses are associated with prolonged cognitive deficits in learning and memory. The clinical significance of these nonclinical findings is not known, and healthcare providers should balance the benefits of appropriate anesthesia in pregnant women, neonates, and young children who require procedures with the potential risks suggested by the nonclinical data [See Warnings and Precautions (5.6), Use in Specific Populations (8.1), and Nonclinical Toxicology (13.2)].
8.5 Geriatric Use
The minimum alveolar concentration (MAC) of SUPRANE decreases with increasing patient age. The dose should be adjusted accordingly. The average MAC for SUPRANE in a 70 year old patient is two-thirds the MAC for a 20 year old patient [See Dosage and Administration (2) Table 1 and Clinical Studies (14.3)].
8.6 Renal Impairment
Concentrations of 1-4% SUPRANE in nitrous oxide/oxygen have been used in patients with chronic renal or hepatic impairment and during renal transplantation surgery.
Because of minimal metabolism, a need for dose adjustment in patients with renal and hepatic impairment is not to be expected.
Nine patients receiving desflurane (N=9) were compared to 9 patients receiving isoflurane, all with chronic renal insufficiency (serum creatinine 1.5-6.9 mg/dL). No differences in hematological or biochemical tests, including renal function evaluation, were seen between the two groups. Similarly, no differences were found in a comparison of patients receiving either desflurane (N=28) or isoflurane (N=30) undergoing renal transplant.
8.7 Hepatic Impairment
Eight patients receiving SUPRANE were compared to six patients receiving isoflurane, all with chronic hepatic disease (viral hepatitis, alcoholic hepatitis, or cirrhosis). No differences in hematological or biochemical tests, including hepatic enzymes and hepatic function evaluation, were seen.
•
Geriatric Use: The minimum alveolar concentration (MAC) of SUPRANE decreases with increasing patient age. (8.5)
DESCRIPTION SECTION
11 DESCRIPTION
SUPRANE (desflurane, USP), a nonflammable liquid administered via vaporizer, is a general inhalation anesthetic. It is (±)1,2,2,2-tetrafluoroethyl difluoromethyl ether:
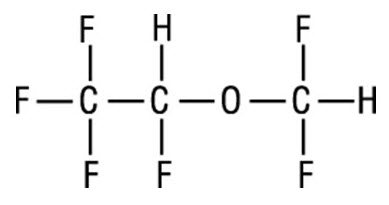
Some physical constants are:
|
Molecular weight |
168.04 |
|
Specific gravity (at 20°C/4°C) |
1.465 |
|
Vapor pressure in mm Hg |
669 mm Hg @ 20°C |
|
731 mm Hg @ 22°C | |
|
757 mm Hg @ 22.8°C (boiling point;1atm) | |
|
764 mm Hg @ 23°C | |
|
798 mm Hg @ 24°C | |
|
869 mm Hg @ 26°C |
Partition coefficients at 37°C:
|
Blood/Gas |
0.424 |
|
Olive Oil/Gas |
18.7 |
|
Brain/Gas |
0.54 |
Mean Component/Gas Partition Coefficients:
|
Polypropylene (Y piece) |
6.7 |
|
Polyethylene (circuit tube) |
16.2 |
|
Latex rubber (bag) |
19.3 |
|
Latex rubber (bellows) |
10.4 |
|
Polyvinylchloride (endotracheal tube) |
34.7 |
SUPRANE is nonflammable as defined by the requirements of International Electrotechnical Commission 601-2-13.
SUPRANE is a colorless, volatile liquid below 22.8°C. Data indicate that SUPRANE is stable when stored under normal room lighting conditions according to instructions.
SUPRANE is chemically stable. The only known degradation reaction is through prolonged direct contact with soda lime producing low levels of fluoroform (CHF3). The amount of CHF3 obtained is similar to that produced with MAC- equivalent doses of isoflurane. No discernible degradation occurs in the presence of strong acids.
SUPRANE does not corrode stainless steel, brass, aluminum, anodized aluminum, nickel plated brass, copper, or beryllium.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.2 Pharmacodynamics
Changes in the clinical effects of SUPRANE rapidly follow changes in the inspired concentration. The duration of anesthesia and selected recovery measures for SUPRANE are given in the following tables:
In 178 female outpatients undergoing laparoscopy, premedicated with fentanyl (1.5- 2.0 µg/kg), anesthesia was initiated with propofol 2.5 mg/kg, desflurane/N2O 60% in O2 or desflurane/O2 alone. Anesthesia was maintained with either propofol 1.5-9.0 mg/kg/hr, desflurane 2.6-8.4% in N2O 60% in O2, or desflurane 3.1-8.9% in O2.
| ||||
|
Emergence and Recovery After Outpatient Laparoscopy | ||||
|
Induction: |
Propofol |
Propofol |
Desflurane/ |
Desflurane/ |
|
Maintenance: |
Propofol/ |
Desflurane/ |
Desflurane/ |
Desflurane/ |
|
Number of Pts: |
N = 48 |
N = 44 |
N = 43 |
N = 43 |
|
Median age |
30 |
26 |
29 |
30 |
|
(20 - 43) |
(21 - 47) |
(21 - 42) |
(20 - 40) | |
|
Anesthetic time |
49 ± 53 |
45 ± 35 |
44 ± 29 |
41 ± 26 |
|
(8 - 336) |
(11 - 178) |
(14 - 149) |
(19 - 126) | |
|
Time to open eyes |
7 ± 3 |
5 ± 2* |
5 ± 2* |
4 ± 2* |
|
(2 - 19) |
(2 - 10) |
(2 - 12) |
(1 - 11) | |
|
Time to state name |
9 ± 4 |
8 ± 3 |
7 ± 3* |
7 ± 3* |
|
(4 - 22) |
(3 - 18) |
(3 - 16) |
(2 - 15) | |
|
Time to stand |
80 ± 34 |
86 ± 55 |
81 ± 38 |
77 ± 38 |
|
(40 - 200) |
(30 - 320) |
(35 - 190) |
(35 - 200) | |
|
Time to walk |
110 ± 6 |
122 ± 85 |
108 ± 59 |
108 ± 66 |
|
(47 - 285) |
(37 – 375) |
(48 - 220) |
(49 - 250) | |
|
Time to fit for |
152 ± 75 |
157 ± 80 |
150 ± 66 |
155 ± 73 |
|
discharge |
(66 - 375) |
(73 - 385) |
(68 - 310) |
(69 - 325) |
In 88 unpremedicated outpatients, anesthesia was initiated with thiopental 3-9 mg/kg or desflurane in O2. Anesthesia was maintained with isoflurane 0.7-1.4% in N2O 60%, desflurane 1.8-7.7% in N2O 60%, or desflurane 4.4-11.9% in O2.
| ||||
|
Emergence and Recovery Times in Outpatient Surgery | ||||
|
Induction: |
Thiopental |
Thiopental |
Thiopental |
Desflurane/ |
|
Maintenance: |
Isoflurane/ |
Desflurane/ |
Desflurane/ |
Desflurane/ |
|
Number of Pts: |
N = 23 |
N = 21 |
N = 23 |
N = 21 |
|
Median age |
43 |
40 |
43 |
41 |
|
(20 - 70) |
(22 - 67) |
(19 - 70) |
(21-64) | |
|
Anesthetic time |
49 ± 23 |
50 ± 19 |
50 ± 27 |
51 ± 23 |
|
(11 - 94) |
(16 - 80) |
(16 - 113) |
(19 - 117) | |
|
Time to open eyes |
13 ± 7 |
9 ± 3* |
12 ± 8 |
8 ± 2* |
|
(5 - 33) |
(4 - 16) |
(4 - 39) |
(4 - 13) | |
|
Time to state name |
17 ± 10 |
11 ± 4* |
15 ± 10 |
9 ± 3* |
|
(6 - 44) |
(6 - 19) |
(6 - 46) |
(5 - 14) | |
|
Time to walk |
195 ± 67 |
176 ± 60 |
168 ± 34 |
181 ± 42 |
|
(124 - 365) |
(101 - 315) |
(119 - 258) |
(92 - 252) | |
|
Time to fit for |
205 ± 53 |
202 ± 41 |
197 ± 35 |
194 ± 37 |
|
discharge |
(153 - 365) |
(144 - 315) |
(155 - 280) |
(134 - 288) |
Recovery from anesthesia was assessed at 30, 60, and 90 minutes following 0.5 MAC desflurane (3%) or isoflurane (0.6%) in N2O 60% using subjective and objective tests. At 30 minutes after anesthesia, only 43% of patients in the isoflurane group were able to perform the psychometric tests compared to 76% in the SUPRANE group (p < 0.05).
| ||||
|
Recovery Tests: Percent of Preoperative Baseline Values | ||||
|
60 minutes After Anesthesia |
90 minutes After Anesthesia | |||
|
Maintenance: |
Desflurane/ |
Isoflurane/ |
Desflurane/ |
Isoflurane/ |
|
Confusion* |
66 ± 6 |
47 ± 8 |
75 ± 7† |
56 ± 8 |
|
Fatigue* |
70 ± 9† |
33 ± 6 |
89 ± 12† |
47 ± 8 |
|
Drowsiness* |
66 ± 5† |
36 ± 8 |
76 ± 7† |
49 ± 9 |
|
Clumsiness* |
65 ± 5 |
49 ± 8 |
80 ± 7† |
57 ± 9 |
|
Comfort* |
59 ± 7† |
30 ± 6 |
60 ± 8† |
31 ± 7 |
|
DSST‡** score** |
74 ± 4† |
50 ± 9 |
75 ± 4† |
55 ± 7 |
|
Trieger Tests§ |
67 ± 5 |
74 ± 6 |
90 ± 6 |
83 ± 7 |
SUPRANE was studied in twelve volunteers receiving no other drugs. Hemodynamic effects during controlled ventilation (PaCO2 38 mm Hg) were:
| ||||||||
|
Hemodynamic Effects of Desflurane During Controlled Ventilation | ||||||||
|
Total MAC Equivalent |
End-Tidal % Des/O****2 |
End-Tidal % Des/N2O |
Heart Rate (beats/min) |
Mean Arterial Pressure (mm Hg) |
Cardiac Index (L/min/m2) | |||
|
O****2 |
N2O |
O****2 |
N2O |
O****2 |
N2O | |||
|
0 |
0% / 21% |
0% / 0% |
69 ± 4 |
70 ± 6 |
85 ± 9 |
85 ± 9 |
3.7 ± 0.4 |
3.7 ± 0.4 |
|
(63 - 76) |
(62 - 85) |
(74 - 102) |
(74 - 102) |
(3.0 - 4.2) |
(3.0 - 4.2) | |||
|
0.8 |
6% / 94% |
3% / 60% |
73 ± 5 |
77 ± 8 |
61 ± 5* |
69 ± 5* |
3.2 ± 0.5 |
3.3 ± 0.5 |
|
(67 - 80) |
(67 - 97) |
(55 - 70) |
(62 - 80) |
(2.6 - 4.0) |
(2.6 - 4.1) | |||
|
1.2 |
9% / 91% |
6% / 60% |
80 ± 5* |
77 ± 7 |
59 ± 8* |
63 ± 8* |
3.4 ± 0.5 |
3.1 ± 0.4* |
|
(72 - 84) |
(67 - 90) |
(44 - 71) |
(47 - 74) |
(2.6 - 4.1) |
(2.6 - 3.8) | |||
|
1.7 |
12% / 88% |
9% / 60% |
94 ± 14* |
79 ± 9 |
51 ± 12* |
59 ± 6* |
3.5 ± 0.9 |
3.0 ± 0.4* |
|
(78 - 109) |
(61 - 91) |
(31 - 66) |
(46 - 68) |
(1.7 - 4.7) |
(2.4 - 3.6) |
When the same volunteers breathed spontaneously during desflurane anesthesia, systemic vascular resistance and mean arterial blood pressure decreased; cardiac index, heart rate, stroke volume, and central venous pressure (CVP) increased compared to values when the volunteers were conscious. Cardiac index, stroke volume, and CVP were greater during spontaneous ventilation than during controlled ventilation.
During spontaneous ventilation in the same volunteers, increasing the concentration of SUPRANE from 3% to 12% decreased tidal volume and increased arterial carbon dioxide tension and respiratory rate. The combination of N2O 60% with a given concentration of desflurane gave results similar to those with desflurane alone. Respiratory depression produced by desflurane is similar to that produced by other potent inhalation agents.
The use of desflurane concentrations higher than 1.5 MAC may produce apnea.
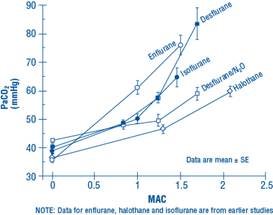
Figure 1. PaCO2 During Spontaneous Ventilation in Unstimulated Volunteers
12.3 Pharmacokinetics
Due to the volatile nature of desflurane in plasma samples, the washin-washout profile of desflurane was used as a surrogate of plasma pharmacokinetics. SUPRANE is a volatile liquid inhalation anesthetic minimally biotransformed in the liver in humans. Less than 0.02% of the desflurane absorbed can be recovered as urinary metabolites (compared to 0.2% for isoflurane). Eight healthy male volunteers first breathed 70% N2O/30% O2 for 30 minutes and then a mixture of desflurane 2.0%, isoflurane 0.4%, and halothane 0.2% for another 30 minutes. During this time, inspired and end-tidal concentrations (FI and FA) were measured. The FA/FI (washin) value at 30 minutes for desflurane was 0.91, compared to 1.00 for N2O, 0.74 for isoflurane, and 0.58 for halothane (see Figure 2). The washin rates for halothane and isoflurane were similar to literature values. The washin was faster for desflurane than for isoflurane and halothane at all time points. The FA/FAO (washout) value at 5 minutes was 0.12 for desflurane, 0.22 for isoflurane, and 0.25 for halothane (see Figure 3). The washout for desflurane was more rapid than that for isoflurane and halothane at all elimination time points. By 5 days, the FA/FAO for desflurane is 1/20th of that for halothane or isoflurane.
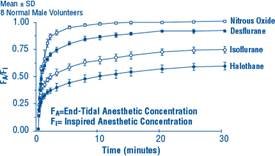
Figure 2. Desflurane Washin
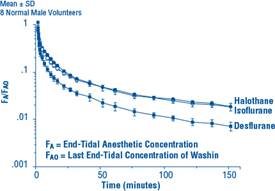
Figure 3. Desflurane Washout
12.5 Pharmacogenomics
RYR1 and CACNA1S are polymorphic genes and multiple pathogenic variants have been associated with malignant hyperthermia susceptibility (MHS) in patients receiving volatile anesthetic agents, including SUPRANE. Case reports as well as ex-vivo studies have identified multiple variants in RYR1 and CACNA1S associated with MHS. Variant pathogenicity should be assessed based on prior clinical experience, functional studies, prevalence information, or other evidence [see Contraindications (4), Warnings and Precautions (5.1)].
