Cabergoline
Cabergoline Tablets
e497366b-a124-4d7f-bd45-a883c392d4bb
HUMAN PRESCRIPTION DRUG LABEL
Nov 28, 2022
Greenstone LLC
DUNS: 825560733
Mylan Pharmaceuticals Inc.
DUNS: 059295980
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
cabergoline
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (3)
Drug Labeling Information
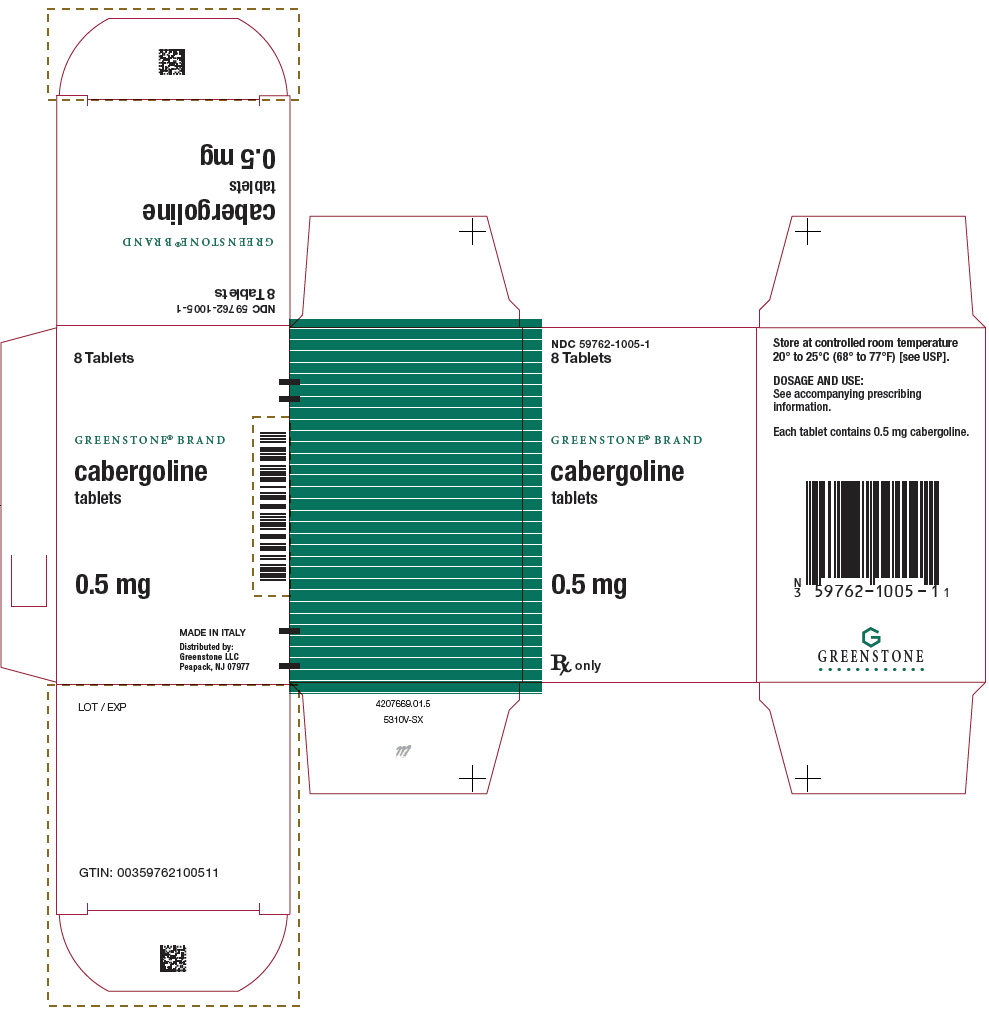
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 8 Tablet Bottle Carton
NDC 59762-1005-1
8 Tablets
GREENSTONE**®**** BRAND**
cabergoline
tablets
0.5 mg
Rx only
SPL UNCLASSIFIED SECTION
Rx only
This product's label may have been updated. For current full prescribing information, please visit www.Greenstonellc.com

LAB-0304-8.0
Revised: November 2019
DESCRIPTION SECTION
DESCRIPTION
CABERGOLINE tablets contain cabergoline, a dopamine receptor agonist. The chemical name for cabergoline is 1-[(6-allylergolin-8β-yl)-carbonyl]-1-[3-(dimethylamino) propyl]-3-ethylurea. Its empirical formula is C26H37N5O2, and its molecular weight is 451.62. The structural formula is as follows:
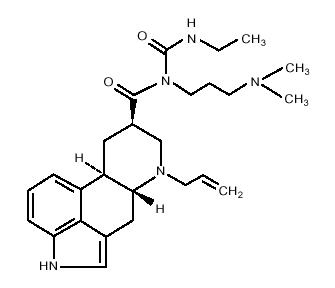
Cabergoline is a white powder soluble in ethyl alcohol, chloroform, and N, N-dimethylformamide (DMF); slightly soluble in 0.1N hydrochloric acid; very slightly soluble in n-hexane; and insoluble in water.
Cabergoline tablets, for oral administration, contain 0.5 mg of cabergoline. Inactive ingredients consist of leucine, USP, and lactose, NF.
