IBUPROFEN
IBUPROFEN 400 MG - 600 MG AND 800 MG TABLETS
9dfc752f-3f66-4c39-bfa8-db6de9a9a9bf
HUMAN PRESCRIPTION DRUG LABEL
Jan 3, 2024
REMEDYREPACK INC.
DUNS: 829572556
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
IBUPROFEN
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Drug Labeling Information
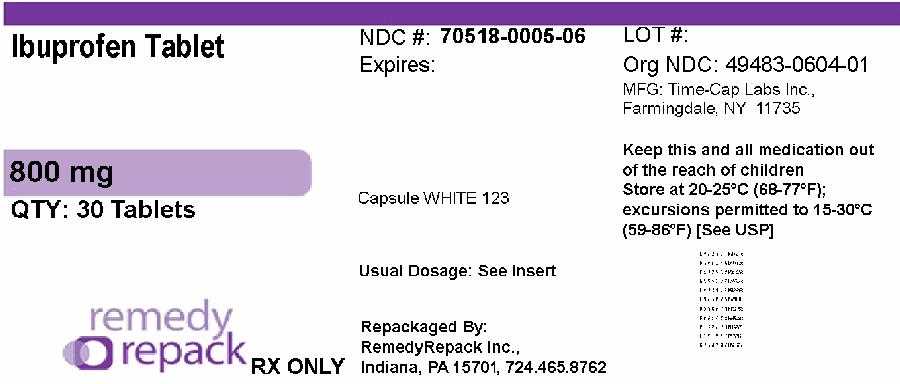
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
DRUG: IBUPROFEN
GENERIC: IBUPROFEN
DOSAGE: TABLET, FILM COATED
ADMINSTRATION: ORAL
NDC: 70518-0005-0
NDC: 70518-0005-1
NDC: 70518-0005-2
NDC: 70518-0005-3
NDC: 70518-0005-4
NDC: 70518-0005-5
NDC: 70518-0005-6
COLOR: white
SHAPE: CAPSULE
SCORE: No score
SIZE: 19 mm
IMPRINT: 123
PACKAGING: 21 in 1 BLISTER PACK
PACKAGING: 14 in 1 BLISTER PACK
PACKAGING: 30 in 1 BLISTER PACK
PACKAGING: 10 in 1 BLISTER PACK
PACKAGING: 90 in 1 BOTTLE PLASTIC
PACKAGING: 30 in 1 BOTTLE PLASTIC
PACKAGING: 30 in 1 BLISTER PACK
ACTIVE INGREDIENT(S):
- IBUPROFEN 800mg in 1
INACTIVE INGREDIENT(S):
- SILICON DIOXIDE
- CROSCARMELLOSE SODIUM
- MAGNESIUM STEARATE
- CELLULOSE, MICROCRYSTALLINE
- POLYETHYLENE GLYCOL, UNSPECIFIED
- POLYVINYL ALCOHOL
- STARCH, PREGELATINIZED CORN
- TALC
- TITANIUM DIOXIDE
HOW SUPPLIED SECTION
Ibuprofen tablets are available in the following strength:
800 mg (white to off white, capsule shaped, biconvex, film coated tablets debossed with ‘123’ on one side and plain on other side)
NDC: 70518-0005-00
NDC: 70518-0005-01
NDC: 70518-0005-02
NDC: 70518-0005-03
NDC: 70518-0005-04
NDC: 70518-0005-05
NDC: 70518-0005-06
PACKAGING: 21 in 1 BLISTER PACK
PACKAGING: 14 in 1 BLISTER PACK
PACKAGING: 30 in 1 BLISTER PACK
PACKAGING: 10 in 1 BLISTER PACK
PACKAGING: 90 in 1 BOTTLE PLASTIC
PACKAGING: 30 in 1 BOTTLE PLASTIC
PACKAGING: 30 in 1 BLISTER PACK
“Preserve in well-closed containers”
Store at 20 ° to 25°C (68°to 77°F) [See USP Controlled Room Temperature].
Rx only
Repackaged and Distributed By:
Remedy Repack, Inc.
625 Kolter Dr. Suite #4 Indiana, PA 1-724-465-8762
SPL MEDGUIDE SECTION
ibuprofen tablets 400 mg - 600 mg- 800 mg medguide

Repackaged By / Distributed By: RemedyRepack Inc.
625 Kolter Drive, Indiana, PA 15701
(724) 465-8762
