FOSFOMYCIN TROMETHAMINE
FOSFOMYCIN TROMETHAMINE granules for oral solution
d101b5d8-bffc-4ee6-b93b-4589fcb8750d
HUMAN PRESCRIPTION DRUG LABEL
Jan 28, 2022
Cipla USA Inc.
DUNS: 078719707
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
FOSFOMYCIN TROMETHAMINE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
DESCRIPTION SECTION
DESCRIPTION
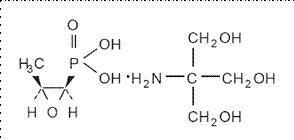
Fosfomycin tromethamine granules for oral solution contains fosfomycin tromethamine, a synthetic, broad spectrum, bactericidal antibiotic for oral administration. It is available as a single-dose sachet which contains white granules consisting of 5.631 grams of fosfomycin tromethamine (equivalent to 3 grams of fosfomycin), and the following inactive ingredients: mandarin flavor, orange powder flavor, saccharin, and sucrose. The contents of the sachet must be dissolved in water. Fosfomycin tromethamine, a phosphonic acid derivative, is available as (1R,2S)-(1,2-epoxypropyl)phosphonic acid, compound with 2-amino-2-(hydroxymethyl)-1,3-propanediol (1:1). It is a white or almost white, hygroscopic powder with a molecular weight of 259.19. Its empirical formula is C3H7O4P.C4H11NO3, and its chemical structure is as follows: