Acetazolamide
Acetazolamide Tablets USP
f7227cc5-0a80-47df-baf8-09f1b22b187e
HUMAN PRESCRIPTION DRUG LABEL
May 19, 2023
Bryant Ranch Prepack
DUNS: 171714327
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Acetazolamide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
Drug Labeling Information
DESCRIPTION SECTION
DESCRIPTION
Acetazolamide, USP an inhibitor of the enzyme carbonic anhydrase, is a white to faintly yellowish white crystalline odorless powder, very slightly soluble in water, sparingly soluble in boiling water and slightly soluble in alcohol. The chemical name for acetazolamide is N-(5-Sulfamoyl-1,3,4-thiadiazol-2-yl)acetamide and has the following chemical structure:
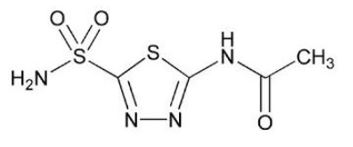
Molecular Weight: 222.25
Molecular Formula: C 4H 6N 4O 3S 2
Acetazolamide, USP is available as oral tablets containing 125 mg and 250 mg of acetazolamide respectively and the following inactive ingredients: lactose monohydrate, magnesium stearate, maize starch, povidone, sodium starch glycolate-type A and talc.
